For long the CNS had been considered to lack lymphatics. The last few years have been spotted with works conflicting the immune-privilege status of CNS. Experimental evidence has assured the presence of lymphatics in the meninges, the meningeal lymphatic vessels (mLVs), along the venous sinuses carrying the immune cells along with drainage of cerebrospinal fluid (CSF). This article aims to explain the findings of multiple studies indicating the existence and clinical importance of the mLVs along with the controversies that refute the concept. A total of 30 studies were included after the search for literature was conducted in three major databases: PubMed, Scopus and Google scholar till through May 2020, with appropriate MESH terms like CNS, lymphatics, meninges, drainage, and glymphatics. Despite evidence supporting the existence of functional lymphatics in CNS, there has been conflict in opinion about the clearance by glymphatic system. Few have countered the CSF clearance mechanism, stating that those experiments showing such finding were performed on post-mortem tissue. The discovery of mLVs emphasizes a re-evaluation of fundamental neuroimmunology theory while enlightening a shift in the aetiology of neuro-inflammatory and neurodegenerative disorders accompanying the dysfunctional immune system.
Meningeal lymphatic vessels: their morphology, location, and clinical implications
Adil Asghar, Ravi K. Narayan, Ashutosh Kumar
Department of Anatomy, AIIMS Patna, India
SUMMARY
Sign up or Login
INTRODUCTION
The lymphatic system implements protein homeostasis and immune surveillance. The classical dogma of the immune-privileged status of the central nervous system (CNS) is gradually diminishing with the discovery and evidence-based explanation of drainage routes for meningeal lymphatics. The previous texts and literature considered the central nervous system devoid of lymphatics and hence no connections to the tonsils, adenoids, and deep cervical lymph nodes (cLNs) were to be considered. This conventional wisdom is now being challenged on the basis of new experimental findings (Louveau et al., 2015; 2017; 2018; Aspelund et al., 2015; Dissing-Olesen et al., 2015; Da Mesquita et al., 2018; Ahn et al., 2019; Chen and Wang, 2020).
Louveau et al. (2015) showed presence of lymphatic channels using immunohistochemical markers in the whole-mount meninges of mouse brain. They observed vessel-like patterns in the endothelial distribution along the sinuses, in which immune cells (T-cell) were linearly aligned and the endothelial linings were positive for the vascular endothelial marker CD31 and classic lymphatic endothelial cell (LEC) markers such as Lyve-1, Prox1, vascular endothelial growth factor receptor 3 (VEGFR3), and the podoplanin. They also provided preliminary data for the presence of Lyve-1 and podoplanin expression in the meningeal coronal sections around the superior sagittal sinus in humans (Louveau et al., 2015; 2017; 2018). Later, Absinta et al. (2017), detected the presence of meningeal lymphatic vessels (mLVs) along the venous sinuses present in the dura, particularly along the straight and superior sagittal sinuses, also along the branches of the middle meningeal artery, in five human volunteers and three marmoset monkeys using image contrast (gadolinium) MRI. These preliminary experiments suggested that meningeal lymphatics exist in humans, which were perhaps unnoticed by earlier studies due to their peculiar anatomical localization. Now accumulative evidence corroborates that these mLVs express the molecular characteristics of LECs and transports the immune cells and fluid from the cerebrospinal fluid (CSF) to the deep cervical lymph nodes (cLNs) (Louveau et al., 2015; 2017; 2018; Aspelund et al., 2015; Ahn et al., 2019; Raper et al., 2016; Absinta et al., 2017).
The search strategy was performed to identify recent information about the localization and function of glympahtics and meningeal lymphatic vessels, as well as novel findings surrounding their role in the pathophysiology of neurodegenerative disease. The search was conducted in three major databases: PubMed, Scopus and Google scholar till through May 2020. The literature was searched after collecting the appropriate MESH terms for meningeal lymphatic vessels and glymphatics. The search strategy used was: ((Glymphatic) AND (cytoplasmic transport) OR (axonal flow)) AND ((efflux) OR lymph flow) OR perineural flow)) AND ((nervous system) OR brain OR cerebrum OR Cerebral OR meningeal fold) AND ((((Human) OR mammal)) AND (meningeal [All Fields] OR dural [All Fields]) AND (“lymphatic vessels”[MeSH Terms] OR (“lymphatic”[All Fields] AND “vessels”[All Fields]) OR “lymphatic vessels”[All Fields] OR “lymphatics”[All Fields] OR “lymphatic system”[MeSH Terms] OR (“lymphatic”[All Fields] AND “system”[All Fields]) OR “lymphatic system” [All Fields)))). A total of 30 studies were collected after removing the duplicates from all three major databases. The articles dealing with the human or rodents or other mammalian brain lymphatics or glymphatics were included in the study, whereas the studies on non-mammalian species were excluded.
ANATOMICAL LOCATION AND MORPHOLOGY OF MENINGEAL LYMPHATICS VESSELS
Louveau et al. (2015) showed that the meningeal lymphatic vessels (mLVs) are localised mostly in the dorsal skull, along the transverse sinus (TS) and superior sagittal sinus (SSS). Ahn et al. (2019) reported mLVs in lateral or basal parts of the skull (basal mLVs) too. They noted that the basal mLVs directed by the side of the sigmoid sinus and petro-squamosal sinus (PSS) were wider and had branch-like, protruding capillaries in abundance (Fig. 2). The characteristic features of blunt-ended capillaries, typical oak-leaf-shaped lymphatic valves, and LECs advocated their similarity to the typical LVs (Fig. 1c). However, the mLVs of the dorsal skull were observed to be inappropriate for carrying and draining the macromolecules of the CSF because of the continuous sealed zipper-like junctional pattern of LECs with underdeveloped morphology (Fig. 1a). Conversely, the branched capillary basal mLVs presented mostly with pre-eminent, sporadically secured loose button-like coupling motif of LECs (Fig. 1c). Around the foramina of the skull, the basal mLVs had more zipper-type couplings with lymphatic valves (Ahn et al., 2019; Papadopoulos et al., 2020).
The peripheral lymphatic vessels (LVs) are categorized into two types: collecting and capillary lymphatic vessels. Dorsal mLVs falls in the category of capillary LVs, which are specialized for the carrying macromolecules and fluid because of characteristics like the lack of the smooth muscle cells (SMCs) and loose button-like LEC junctions. Basal mLVs present with amalgamated attributes of both collecting and capillary LVs, marked by diversity in the junctional/ coupling patterns and by the absence of SMCs (Ahn et al., 2019). Due to the presence of valves, basal mLVs are considered as ‘pre-collecting mLVs’ instead of ‘collecting mLVs’ (which possess compact zipper-like LEC junctions along with SMCs and a lymphatic valve per lymphangion segment). The characteristic features of basal mLVs enables them to commit for the dual role of uptake and transport of CSF (Da Mesquita et al., 2018; Chen and Wang, 2020).
DRAINING CSF THROUGH THE BASAL OUTFLOW
In many different species, Schwalbe (1869) stipulated a part for the LVs in drainage of CSF in the regions of both cranium and spinal cord (Yang et al., 2013). Boulton et al. (1998) injected a radiolabel tracer in CSF and tried to quantify extracranial drainage of the CSF by cannulating lymphatic vessels of the deep cervical region in sheep. Similar evidence was earlier presented by Bradbury and Westrop (1983) in rabbits. These studies suggested that approximately 30-50% of the CSF outflow occurs via LVs along the sheaths of cranial nerves and the cribriform plate (nasal mucosa & olfactory nerve; Fig. 1b), while the rest is presumed to have drained via arachnoid villi. Ma et al. (2017) showed in mice that the LVs were the most significant pathway for drainage of both small molecule tracers from the CSF and macromolecules.17
The mLVs present a draining channel for the interstitial fluid (ISF) and CSF into the cervical lymph nodes (cLNs) (Absinta et al., 2017; Antila et al., 2017; Dupont et al., 2020). Aspelund et al. (2015) and Louveau et al. (2015) illustrated in mice models that mLVs exhibits a significant part in clearing the solute of ISF and CSF (Louveau et al., 2018; Absinta et al., 2017). Both of them have given stress on dorsal mLVs, but were silent on the basal mLVs. Ahn et al. (2019) showed in mice that drainage of CSF is primarily done by mLVs present at the cranial base. After injecting the contrast into the lateral ventricle of mice brain with stable vital signs, a swift escalation in contrast enhancement was observed initially in the lateral ventricle on the right, and then in basal outflow near the skull foramina, especially the jugular foramen, and finally the contrast reached the deep cervical LNs (Louveau et al., 2018). They explained that, as the branched capillary basal mLVs are detected within a much thinner layer of the dura near the subarachnoid space, and separated from that by a loose arachnoid barrier as compared to the dorsal mLVs, so basal mLVs were more likely to be facilitating the carriage and transport of CSF than dorsal mLVs.
Although these animal studies strongly suggest for similar scenarios in humans, a direct evidence in humans is still lacking. Besides, none of the studies have yet commented on the role of physiological and diurnal variations in mLVs-mediated clearance of CSF.
THE LINK BETWEEN THE MENINGEAL LYMPHATIC VESSELS AND ‘GLYMPHATIC SYSTEM’
The ‘glymphatic system’ can be explained as a CSF/lymphatic system dependent on glial cells present all over the brain, comprising of a meshwork of para-vascular spaces that permits the systematic disposal of wastes and interstitial solutes. Well-described Virchow-Robin spaces (VRS) are being appraised to be the initiation point for the glymphatic system (Iliff et al., 2013; Louveau et al., 2017). The clearance of CSF by the glymphatic system implies a unique mechanism. From the subarachnoid spaces the CSF is first pushed into the para-arterial spaces (via trans-parenchymal swapping in extracellular tissue), thereafter portals out via para-venous spaces to get back in subarachnoid compartments. Evidently, coupling of para-arterial CSF influx to para-venous ISF clearance within the brain by Aquaporin-4-dependent astroglial water fluxes suggests that the glymphatic pathway interact with the mLVs along the perivascular spaces (Iliff et al., 2012).
There is a conflict in opinion about the clearance by glymphatic system. Few have countered the CSF clearance mechanism stating that those experiments showing such finding were performed on post-mortem tissue. Some of them claimed that minute openings were present in pia mater covering the arteries, which provided an anatomical route for macromolecules and CSF to be taken into the para-vascular space via the basal cistern. Nedergaard and colleagues explained the route in which AQP4-dependent convection in brain parenchyma is required for transferring of solutes from CSF to ISF. The consistent evidence explains parenchymal diffusion in the paravascular spaces via either dispersive or convective flow. It is sufficient to explain movement of solutes in the brain (Asgari et al., 2016; Jin et al., 2016). The authors who oppose the glymphatic mechanism claimed that CSF circulation is upregulated in awake state and downregulated in sleep. They argued for re-evaluation of Aquaporin-4-dependent parenchymal and para-vascular waste clearance and asked to study the redistribution of solute in para-vascular space after deletion of Aquaporin-4 channel from astrocyte foot process (Smith et al., 2017). CSF circulation may be decreased in sleep state but no study refutes the claim of high glymphatic efflux in sleep state and anesthetized rodents.
ROLE OF MLVS IN NEURO-IMMUNOLOGY AND DISEASES
The discovery of mLVs emphasizes a re-evaluation of fundamental theory in neuroimmunology while enlightening a shift in the aetiology of neuro-inflammatory and neurodegenerative disorders accompanying the dysfunctional immune system. The meningeal lymphatics are now thought to be the major route for clearance of macromolecules such as alpha-synuclein in Parkinson’s disease, amyloid body in Alzheimer’s disease, multisystem atrophy, Lewy body disease, and all prion-like proteinopathies, and demyelinating disease like multiple sclerosis (Iliff et al., 2012; Jaffe et al., 2019; Song et al., 2020; Pal et al., 2020; Balint et al., 2020; Frederick and Louveau, 2020).
The distension of the dural mLVs and the accumulation of T lymphocytes in mice occurs when collecting vessels draining to the deep cervical lymph nodes are ligated. This indicates that these vessels play a part in presentation of antigen and immune surveillance of the CNS (Louveau et al., 2015). This decoding of the mechanism by which T cells may participate in immuno-surveillance of the CNS might be of much help in therapeutically altering the susceptibility of the brain to viral infections in immunocompromised individuals (HIV+ patients and transplant recipients) (Iliff et al., 2012).
Apart from the above-mentioned entities, the mLVs may also be involved in abnormal protein drainage from the brain tumours into the lymph nodes, thus triggering an explosive immune response (Iliff et al., 2012). Having realized that the CNS has its own lymphatic drainage, we can now shift our thinking back to the yet unexplained pathophysiology of the immune-cell-mediated brain disorders, as well as the effects of these lymphatics on drainage of subdural haematomas and the effect of damage to these draining channels on traumatic brain injury (Hershenhouse et al., 2019; Liu et al., 2020).
It is necessary to emphasize that functional studies of mLVs have been restricted mostly to the animal models. Further research in human models will provide new insight in the future by explaining therapeutically exploitable etiological relations between the brain’s fluid dynamics and its disorders. Further, the mapping of the complete meningeal lymphatic vessels, including glial-lymphatic system, will be required. Better understanding of the detailed anatomy of the meningeal vessels in human will propel the interventions to halt the progression of immune-mediated brain diseases where these vessels may plausibly be involved.
Related articles
 Fig. 1.- a – showing dorsal mLVs, which are thin, discontinuous, valve less and have zipper-like LEC junctions; b – mid-sagittal section of the cranium with a green line in between the dural sinuses representing mLVs. Dorsal mLVs run along either sides of the superior sagittal sinus (SSS) and drain to the deep cLNs via perineural sheath across the cribriform plate, while the basal mLVs runs along the sigmoid sinus (SS) and also drain to the deep cLNs after exiting the cranial cavity via jugular foramen; c – morphology of basal mLVs, which have larger diameter with valves, blunt end capillaries and button like LEC junctions.
Fig. 1.- a – showing dorsal mLVs, which are thin, discontinuous, valve less and have zipper-like LEC junctions; b – mid-sagittal section of the cranium with a green line in between the dural sinuses representing mLVs. Dorsal mLVs run along either sides of the superior sagittal sinus (SSS) and drain to the deep cLNs via perineural sheath across the cribriform plate, while the basal mLVs runs along the sigmoid sinus (SS) and also drain to the deep cLNs after exiting the cranial cavity via jugular foramen; c – morphology of basal mLVs, which have larger diameter with valves, blunt end capillaries and button like LEC junctions.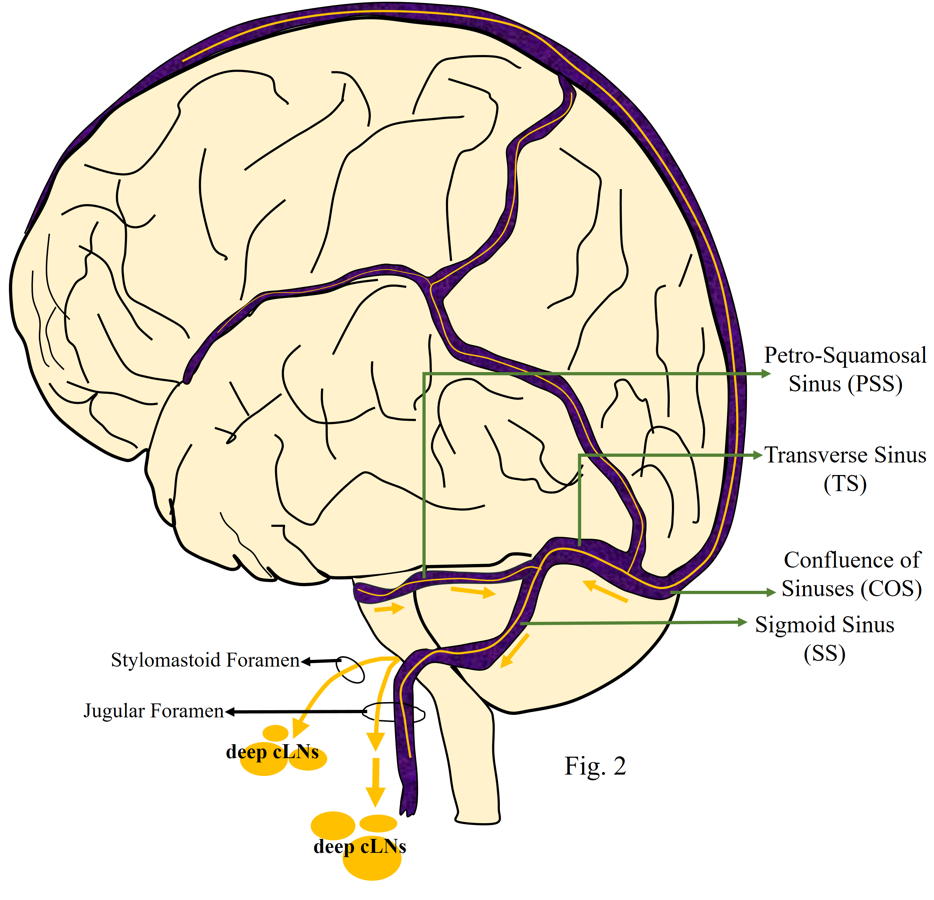 Fig. 2.- Dural venous sinuses in relation to the brain with a green line running between the dural sinuses, representing mLVs. Green arrows are representing flow of the fluid along the basal mLVs which are running along, transverse sinus (TS), petro-squamosal sinus (PSS) and sigmoid sinus (SS). Some of the basal mLVs exit the cranial cavity via stylomastoid foramen, while others exit via jugular foramen and drain into deep cervical lymph nodes (cLNs).
Fig. 2.- Dural venous sinuses in relation to the brain with a green line running between the dural sinuses, representing mLVs. Green arrows are representing flow of the fluid along the basal mLVs which are running along, transverse sinus (TS), petro-squamosal sinus (PSS) and sigmoid sinus (SS). Some of the basal mLVs exit the cranial cavity via stylomastoid foramen, while others exit via jugular foramen and drain into deep cervical lymph nodes (cLNs).ABSINTA M, HA S-K, NAIR G, SATI P, LUCIANO NJ, PALISOC M, LOUVEAU A, ZAGHLOUL KA, PITTALUGA S, KIPNIS J, REICH RS (2017) Human and nonhuman primate meninges harbor lymphatic vessels that can be visualized noninvasively by MRI. Elife, 6: e29738.
AHN JH, CHO H, KIM J-H, KIM SH, HAM J-S, PARK I, SUH SH, HONG SP, SONG J-H, HONG Y-K, JEONG Y, PARK S-H, KOH GY (2019) Meningeal lymphatic vessels at the skull base drain cerebrospinal fluid. Nature, 572(7767): 62-66.
ANTILA S, KARAMAN S, NURMI H, AIRAVAARA M, VOUTILAINEN MH, MATHIVET T, CHILOV D, LI Z, KOPPINEN T, PARK JH, FANG S (2017) Development and plasticity of meningeal lymphatic vessels. J Exp Med, 214(12): 3645-3667.
ASGARI MM, WANG W, IOANNIDIS NM, ITNYRE J, HOFFMANN T, JORGENSON E, WHITTEMORE AS (2016) Identification of susceptibility loci for cutaneous squamous cell carcinoma. J Invest Dermatol, 136(5): 930-937.
ASPELUND A, ANTILA S, PROULX ST, KARLSEN TV, KARAMAN S, DETMAR M, WIIG H, ALITALO K (2015) A dural lymphatic vascular system that drains brain interstitial fluid and macromolecules. J Exp Med, 212(7): 991-999.
BÁLINT L, OCSKAY Z, DEÁK BA, ARADI P, JAKUS Z (2020) Lymph flow induces the postnatal formation of mature and functional meningeal lymphatic vessels. Front Immunol, 10: 3043.
BOULTON M, FLESSNER M, ARMSTRONG D, HAY J, JOHNSTON M (1998) Determination of volumetric cerebrospinal fluid absorption into extracranial lymphatics in sheep. Am J Physiol, 274(1): R88-96.
BRADBURY MW, WESTROP RJ (1983) Factors influencing exit of substances from cerebrospinal fluid into deep cervical lymph of the rabbit. J Physiol (Lond), 339: 519-534.
CHENG Y, WANG YJ (2020) Meningeal lymphatic vessels: a drain of the brain involved in neurodegeneration? Neurosci Bull, 36(5): 557-560.
DA MESQUITA S, FU Z, KIPNIS J (2018) The meningeal lymphatic system: a new player in neurophysiology. Neuron, 100(2): 375-388.
DISSING-OLESEN L, HONG S, STEVENS B (2015) New brain lymphatic vessels drain old concepts. EBioMedicine, 2(8): 776-777.
DUPONT G, IWANAGA J, YILMAZ E, TUBBS RS (2020) Connections between amyloid beta and the meningeal lymphatics as a possible route for clearance and therapeutics. Lymphat Res Biol, 18(1): 2-6.
FREDERICK N, LOUVEAU A (2020) Meningeal lymphatics, immunity and neuroinflammation. Curr Opinion Neurobiol, 62: 41-47.
HERSHENHOUSE KS, SHAULY O, GOULD DJ, PATEL KM (2019) Meningeal lymphatics: a review and future directions from a clinical perspective. Neurosci Insights, 14: 1179069519889027.
ILIFF JJ, WANG M, LIAO Y, PLOGG BA, PENG W, GUNDERSEN GA, BENVENISTE H, VATES GE, DEANE R, GOLDMAN SA, NAGELHUS EA, NEDERGAARD M (2012) A paravascular pathway facilitates CSF flow through the brain parenchyma and the clearance of interstitial solutes, including amyloid β. Sci Transl Med, 4(147): 147ra111.
ILIFF JJ, LEE H, YU M, FENG T, LOGAN J, NEDERGAARD M, BENVENISTE H (2013) Brain-wide pathway for waste clearance captured by contrast-enhanced MRI. J Clin Invest, 123(3): 1299-1309.
JAFFE RJ, DAVE RS, BYRAREDDY SN (2019) Meningeal lymphatics in aging and Alzheimer’s disease. Ann Transl Med, (Suppl 1): S2.
JIN B-J, SMITH AJ, VERKMAN AS (2016) Spatial model of convective solute transport in brain extracellular space does not support a “glymphatic” mechanism. J Gen Physiol, 148(6): 489-501.
LIU X, GAO C, YUAN J, XIANG T, GONG Z, LUO H, JIANG W, SONG Y, HUANG J, QUAN W, WANG D (2020) Subdural haematomas drain into the extracranial lymphatic system through the meningeal lymphatic vessels. Acta Neuropathol Commun, 8(1): 1-1.
LOUVEAU A, SMIRNOV I, KEYES TJ, ECCLES JD, ROUHANI SJ, PESKE JD, DERECKI NC, CASTLE D, MANDELL JW, LEE KS, HARRIS TH, KIPNIS J (2015) Structural and functional features of central nervous system lymphatic vessels. Nature, 523(7560): 337-341.
LOUVEAU A, PLOG BA, ANTILA S, ALITALO K, NEDERGAARD M, KIPNIS J (2017) Understanding the functions and relationships of the glymphatic system and meningeal lymphatics. J Clin Invest, 127(9): 3210-3219.
LOUVEAU A, HERZ J, ALME MN, SALVADOR AF, DONG MQ, VIAR KE, HEROD SG, KNOPP J, SETLIFF JC, LUPI AL, DA MESQUITA S, FROST EL, GAULTIER A, HARRIS TH, CAO R, HU S, LUKENS JR, SMIRNOV I, OVERALL CC, OLIVER G, KIPNIS J (2018) CNS lymphatic drainage and neuroinflammation are regulated by meningeal lymphatic vasculature. Nat Neurosci, 21(10): 1380-1391.
MA Q, INEICHEN BV, DETMAR M, PROULX ST (2017) Outflow of cerebrospinal fluid is predominantly through lymphatic vessels and is reduced in aged mice. Nat Commun, 8(1): 1434.
PAL S, RAO S, LOUVEAU A (2020) Meningeal lymphatic network: The middleman of neuroinflammation. Clin Exp Neuroimmunol, 11(1): 21-25.
PAPADOPOULOS Z, HERZ J, KIPNIS J (2020) Meningeal lymphatics: from anatomy to central nervous system immune surveillance. J Immunol, 204(2): 286-293.
RAPER D, LOUVEAU A, KIPNIS J (2016) How do meningeal lymphatic vessels drain the CNS? Trends Neurosci, 39(9): 581-586.
SMITH AJ, YAO X, DIX JA, JIN B-J, VERKMAN AS (2017) Test of the “glymphatic” hypothesis demonstrates diffusive and aquaporin-4-independent solute transport in rodent brain parenchyma. Elife, 21: 6.
SONG E, MAO T, DONG H, BOISSERAND LS, ANTILA S, BOSENBERG M, ALITALO K, THOMAS JL, IWASAKI A (2020) VEGF-C-driven lymphatic drainage enables immunosurveillance of brain tumours. Nature, 577(7792): 689-694.
YANG L, KRESS BT, WEBER HJ, THIYAGARAJAN M, WANG B, DEANE R, BENVENISTE H, ILIFF JJ, NEDERGAARD M (2013) Evaluating glymphatic pathway function utilizing clinically relevant intrathecal infusion of CSF tracer. J Trans Med, 11(1): 107.