Intramuscular injections administered in the deltoid region must be performed in the denser part of the muscle in an area below the acromion to the deltoid tuberosity. Despite being the most used region, it is the one that presents the greatest risk to the patient’s integrity. Thus, the aim of this work was to analyze the morphology and morphometry of the deltoid region and to establish correlations with neurovascular structures. Thirty upper limbs of adult human cadavers were fixed in formalin solution and subsequently dissected. Parameters as length, width, thickness and distance between the neurovascular bundle and intramuscular injection point were verified using a digital caliper. It was observed that in 100% of the limbs the deltoid muscle presented three multipennate bellies. Lateral margin length (16.71 ± 1.21 cm) was significantly lower compared to that of the anterior (18.69 ± 1.84 cm) and posterior (20.14 ± 2.08 cm) margins. Anterior belly thickness (1.62 ± 0.59 cm) was lower than that of the posterior (2.03 ± 0.53 cm) and similar to that of the lateral belly (1.75 ± 0.41 cm). The total width of the muscle was 12.87 ± 1.97 cm, while the distance from the neurovascular bundle to the intramuscular injection point was 2.85 ± 1.16 cm. There was no morphometric difference between the antimeres, except for the anterior belly thickness, with higher value in the right antimere. It can be concluded that the neurovascular bundle is located in the posterior and posterolateral parts of the muscle and, therefore, within the intramuscular injection area. Thus, the most anterior part of the lateral belly and the whole anterior belly seem to be more favorable sites for administering intramuscular injections.
Morphology and morphometry of the deltoid region applied to intramuscular injections
Bruna Garbin de Souza1, Lázaro A. dos Santos2, Lorena T. de Menezes2, Roberto Bernardino Júnior2, Daniela C. de Oliveira Silva2
1 Course of Nursing, Faculty of Medicine, Federal University of Uberlândia, Uberlandia, MG, Brazil
2 Department of Human Anatomy, Institute of Biomedical Sciences, Federal University of Uberlândia, Uberlandia, MG, Brazil
SUMMARY
Sign up or Login
INTRODUCTION
Since the 1970s, research in nursing regarding the various forms of injections has been developed to investigate various factors, such as complications, better administration sites, procedures to reduce pain and techniques for applying intramuscular injections (Keen, 1986; Beecroft and Redick, 1989; Losek and Gyuro, 1992; Nicoll and Hesby, 2002; Coskun et al., 2016). At this time, intramuscular injections are used frequently and its safe implementation is the nurse’s responsibility (Small, 2004; Coskun et al., 2016). For this purpose, the anatomical knowledge, which constitutes an area of morpho-functional study, provides theoretical support for the various structures of the organism as well as its function, contributing to the success of the technique performed by health professionals (Da Silva and Vaz Vidal, 2013).
The intramuscular route is the best one of administration for antibiotics, corticosteroids and similar drugs in hospital environments, as well for vaccines in primary care centers. Intramuscular injection is chosen for situations in which the absorption rate of a drug is very slow by the subcutaneous route or very fast by the intravenous route (Hopkins and Arias, 2013; Coskun et al., 2016). When performing an injectable administration in the muscle belly, the nurse must be aware of the general factors of anatomical variation including biotype, age, sex, race and local conditions, which follow the characteristics of individual variations in the regions of clinical and therapeutic implications, such as: shape, length, width, thickness and disposition of muscle fibers (Da Silva and Vaz Vidal, 2013). Several studies indicate that intramuscular injections have disadvantages that must be considered, such as abscess formation, erythema, infiltrations in the subcutaneous tissue, embolisms and nerve injuries (Greenblatt and Allen, 1978; Valderrama and Miguel, 1981; Thomaz and Baltar, 1988; Mcivor et al., 1991; Cassiani et al., 1998). Other complications that have been reported include violent pain, radiated or not, during or immediately after the application of the medication, flushing, hematomas, nodules, paresis, paralysis or necrosis (Cassiani et al., 1998).
The main regions selected for intramuscular drug application in the patient and with the greatest clinical implication in the cranio-podalic sense are the deltoid, dorsogluteal and ventrogluteal regions, and the lateral aspect of the thigh (Da Silva and Vaz Vidal, 2013). Among these, the deltoid region is used for the administration of small volume non-irritating drugs, such as vaccines (hepatitis A and B), analgesics, antiemetics, antibiotics and antipsychotics (Mcgarvey and Hooper, 2005; Cocoman and Murray, 2008). Some authors state that administration in this region has been avoided by health professionals due to the higher percentage of complications resulting from muscle punctures when compared to other regions, in addition to its great anatomical disadvantage (Godoy et al., 2004; Meireles and Motta-Filho, 2004; Da Silva and Vaz Vidal, 2013). Other disadvantages that this region can offer to the patient include the small tissue reservoir to absorb the medication, which makes it impossible to introduce large volumes in the muscle belly; the intolerance to irritable substances; and the possibility of injuries to vascular and nervous branches that can seriously compromise their irrigation and drainage as well as the motor function of the arm (Giovani, 2006; Da Silva and Vaz Vidal, 2013).
The “deltoid” injection site is so called because it has the shape of the Greek letter “delta”, which means “V-shaped triangle” (Cocoman and Murray, 2008). The main structure forming this site is a muscle of the same name, the deltoid, a thick and strong muscle, with rough texture, which covers the shoulder and forms its rounded profile. The deltoid muscle is divided in anterior and posterior semipennate parts and a middle multipennate part. The muscle parts can act separately or together. Its proximal fixation is on the lateral third of the clavicle, the acromion and the scapula spine; its distal fixation is on the deltoid tuberosity of the humerus (Moore et al., 2019).
The deltoid muscle is irrigated by the deltoid branch of the thoracoacromial artery, a wide and short trunk that bifurcates from the 2nd part of the axillary artery; and the posterior circumflex humeral artery, which originates from 3rd part of the axillary artery and crosses medially the posterior wall of the axilla through the quadrangular space. Together with the arteries, the posterior circumflex humeral and cephalic veins drain the deltoid muscle; the latter follows over the deltoid muscle along the delto-pectoral groove, between the deltoid and the pectoralis major muscles. The innervation of the deltoid muscle is done by branches of the axillary nerve, which is the terminal branch of the posterior fascicle of the brachial plexus, receiving fibers from C5 and C6; these branches are the upper lateral cutaneous nerve of the arm, which spirals around the surgical neck of the humerus deep into the deltoid muscle, innervating the skin over the lower region of the muscle; and the anterior and posterior branches of the axillary nerve (Moore et al., 2019).
Similar to all intramuscular injections, those injected into the deltoid muscle must be performed in the densest part of the muscle. This part is located by drawing an imaginary horizontal line of two to three finger widths, 2 to 5 cm below the lower margin of the acromial process (Craven and Hirnle, 2008; Rodger and King, 2000). The injection must be applied into an imaginary triangle, whose base is the central half of this horizontal line, and the inverted apex is formed at the midpoint of the lateral aspect of the arm in line with the axilla (Kozier et al., 1993; Rodger and King, 2000).
Considering the possibility of individual anatomical variations that can result in lesions of some structures of the neurovascular bundle when intramuscular injections are administered in the deltoid region (Castellanos, 1977), morphological and morphometric investigations of this region are necessary. In that sense, morphology studies the organic forms, emphasizing the arrangement of anatomical structures belonging to each system and region of the body, while the morphometry investigates the shape variation of the organisms, through correlations between measures of distance and size of body structures (Mandarim-Lacerda, 1995).
Within this context, this research aimed to describe the morphology of the deltoid muscle in human cadavers, evidencing the shape, proximal and distal fixations (origin and insertion) and muscle fibers disposition, as well as the path and distribution of their neurovascular structures. In addition, measurements of the upper limb length, as well as the length, width and thickness of the deltoid muscle and the distance from the neurovascular bundle to the intramuscular injection point were quantified in order to establish comparisons and correlations between the different parameters analyzed, and between right and left antimeres.
MATERIALS AND METHODS
Study design and sample
The research on the morphology and morphometry of the deltoid region applied to intramuscular injections is an experimental, descriptive, quantitative and analytical study, and was performed from January to December 2019.
Thirty upper limbs of adult human cadavers of unknown sex, coming from the laboratory of human anatomy, were used and fixed in 10% formalin solution through subcutaneous, intravenous, intramuscular and intracavitary injections, and kept immersed in containers containing the same solution.
As inclusion criteria in the study, the upper limbs should be healthy, with all segments (shoulder, arm, forearm and hand) present, and the deltoid region intact, especially the deltoid muscle. Exclusion criteria involved upper limbs with missing clavicles, damaged deltoid muscles or neurovascular bundle, or any other injury to the deltoid region.
Data collection instruments
In order to proceed with morphological description and morphometric analysis, all the cadaveric pieces were dissected using a scalpel handle number 4, blades number 23 and 24, straight rat tooth tweezer (16 cm), straight serrated tweezer (16 cm), straight Metzembaum scissors (14 cm), straight Mayo Stille scissors (15 cm) and straight fine/blunt surgical scissor (15 cm).
The parameters of upper limb length as well as length, width and thickness of the deltoid muscle and the distance from the neurovascular bundle to the intramuscular injection point were measured using cotton string (number 6, thickness 4/8, raw), stainless steel pins with round plastic head (5 cm length) and a universal digital caliper, 12”, 300mm, 0.01mm resolution, Zaas Precision (Tecnoferramentas Comercial, Importação e Exportação Ltda, São Paulo, Brazil). The neurovascular bundle was located using a disposable hypodermic needle (model 25x0.8mm, Labor Import) and a syringe (10 ml, Descarpack Luer Slip).
Procedures
The preparation of the anatomical pieces followed the usual dissection techniques and procedures (Rodrigues, 2010). First, the cadaver was placed in a prone position, with the arm adducted at 45º, using a wooden block. The skin and adipose tissue of the back, thoracic region and upper limb were removed; for this, a transverse incision was made on the middle third of the neck, connecting the posterior to the anterior median line. Then, two sagittal sections were performed on the posterior and anterior median lines, extending from the initial incision to the level of the xiphoid process. A fourth incision was made transversally from the posterior to anterior median line at the level of the xiphoid process. After removing the skin, the fasciae were removed and it was possible to visualize and identify the shoulder muscles.
The deltoid muscle margins were defined, with regards to the origin in the lateral third of the clavicle, acromion and scapula spine. The muscle was sectioned from the scapula spine and the acromion by using a scalpel, leaving it attached only in the clavicle. Then, the deltoid muscle was removed towards the anterior plane; the margins of the quadrangular and triangular spaces were defined and the neurovascular structures were exposed and dissected. All structures were prepared with no magnifying glasses and, when necessary, a 10-times magnifying glass was used. Once dissected, the pieces were washed in water for 24 hours in order to remove excess formaldehyde and facilitate the appreciation of the material, which was stored in water containers.
Morphological characteristics of the proximal and distal fixations, shape and topographic relationships of the deltoid muscle were described, as well as origin, path and distribution of neurovascular structures within the muscle.
Morphometric analysis was quantified according to the parameters described in Table 1. The reference points for the parameter measurements of the upper limb length (ULL - sum of arm length-AL and forearm length-FL), muscle length (ML), belly width (BW) and the distance between the neurovascular bundle and the intramuscular injection point (NBII) were marked with the aid of string and pins (Fig. 1A-F, H). Belly thickness (BT) was measured by using the pin, which was inserted deeply until reaching the humerus bone (Fig. 1G), and then, using a pen, a mark was made on it. After each measurement carried out with the string, it was placed on a flat surface, together with the pins, and thus the distance between the points was calculated using the caliper (Fig. 1I). For the BT parameter, the pin was positioned on a flat surface and the measurement was obtained with the caliper (Fig. 1J).
In order to locate the neurovascular bundle (NB), the deltoid posterior belly was removed and the axillary nerve, together with the posterior circumflex humeral artery and vein, were dissected (Fig. 2A). The NB reference point was obtained by crossing the needle with the aid of a syringe from the surface of the deltoid posterior belly to the point of contact with the NB (Fig. 2B).
The reference point of the intramuscular injection was located using the string, which was placed at a distance of three fingers below the lower margin of the acromion angle, forming the base of a triangle, whose apex was located at a distance of one finger above the deltoid tuberosity; the needle with the syringe was inserted in the center of this triangle. Thereafter, the distance between the NBII was measured (Fig. 1H).
Data analysis
All data were analyzed by means of descriptive and analytical statistics, using the Graph Pad Prism 6 program (San Diego, USA). In the descriptive phase, the mean, standard deviation, median and amplitude (minimum and maximum) of each parameter were analyzed; simple percentages were calculated and compared between the means. In the analytical phase, the mean values of muscle length and belly thickness were compared between the anterior, lateral and posterior parts using paired non-parametric tests as the Friedman test and Dunn’s multiple comparison post-test. The mean values of all parameters were compared between antimeres (right and left) using Student t or Mann-Whitney test, when appropriate. Correlations were performed between the parameters of length, thickness and width of muscle bellies or margins, using the Pearson or Spearman correlation coefficient test, when appropriated. Differences were considered statistically significant when p < 0.05.
RESULTS
Regarding the morphological characteristics, in 100% of the dissected upper limbs the deltoid muscle showed bulky with coarse texture and surrounded glenohumeral articulation on all sides except inferomedially, lending the shoulder its rounded profile (Fig. 3A-C). The muscle presented three distinct parts (bellies) with a multipennate aspect, named according to the place of origin of the muscle fibers: (1) anterior or clavicular: anterior border and superior surface of the lateral third of the clavicle (Fig. 3A); (2) lateral or acromial: lateral margin and superior surface of the acromion (Fig. 3B); (3) posterior or scapular: lower edge of the crest of the scapular spine (Fig. 3C). All three parts converged inferiorly to a short tendon, which was attached to the deltoid tuberosity on the lateral aspect of the humerus. The clavicular and scapular parts showed two intramuscular septa, while the acromial part, larger than the first, showed four intramuscular septa that descended from the acromion to interdigitate with three septa ascending from the deltoid tuberosity (Fig. 3A-C). In all the pieces analyzed, the deltoid muscle was supplied by acromial and deltoid branches of the thoracoacromial artery, the anterior and posterior circumflex humeral arteries, subscapular artery and the deltoid branch of the profunda brachii artery; its innervation occurred through the axillary nerve (Fig. 3D).
Table 2 shows the morphometric analysis with descriptive statistics showing the values of mean, standard deviation, median and amplitude of the analyzed parameters obtained from the upper limb and the deltoid muscle. Regarding the length of the anterior, lateral and posterior margins, it was observed that the mean values of PML (20.14 ± 2.08 cm), AML (18.69 ± 1.84 cm) and LML (16.71 ± 1.21 cm) corresponded to 34.38%, 31.91% and 28.52% of the ULL value (58.57 ± 2.32 cm), respectively. Considering the thickness of the anterior, lateral and posterior bellies, the mean values of PBT (2.03 ± 0.53 cm), LBT (1.75 ± 0.41 cm) and ABT (1.62 ± 0.59 cm) represented 15.77%, 13.59% and 12.58% of the BW (12.87 ± 1.97 cm), respectively. The distance from the neurovascular bundle to the intramuscular injection point (NBII) showed a mean value of 2.85 ± 1.16 cm, ranging between 0.63 and 5.11 cm (Table 2).
Figure 4 shows the analytical statistics with comparison of the mean values of the length and thickness morphometric parameters of the deltoid muscle. The mean value of LML (16.71 cm ± 1.21 cm) was significantly lower compared to that of AML (18.69 cm ± 1.84 cm, p < 0.01) and PML (20.14 cm ± 2.08 cm, p < 0.001), with no significant difference between the AML and PML values (Fig. 4A). On the other hand, the mean value of ABT (1.62 cm ± 0.59 cm) was statistically lower than PBT (2.03 cm ± 0.53 cm, p < 0.001) and similar to LBT (1.75 cm ± 0.41 cm) values, with no significant differences between LBT and PBT values (Fig. 4B).
Comparison of the mean values of all morphometric parameters obtained from the upper limb and the deltoid muscle between the right and left antimeres is shown in Fig. 5. Statistically significant difference was observed only between the mean value of the right and left ABT (p = 0.0433) (Fig. 5C). For the other parameters, there were no significant differences (p > 0.05).
Correlations between the selected parameters are shown in Table 3. A positive correlation was found only between the parameters ULL and LML (r: 0.4661, p = 0.0288), BW and ABT (r: 0.3973, p = 0.0328) and NBII and ABT (r: 0.5275, p = 0.0081) (Fig. 6). All other parameters showed no significant correlation (p > 0.05).
DISCUSSION
In this study, the morphology and morphometry of the deltoid region of human cadavers, as well as the path and distribution of its neurovascular structures were described and analyzed. In addition, this is a pioneer study for measuring the distance from the neurovascular bundle to the intramuscular injection point. Therefore, this research demonstrates relevant information regarding the anatomical and morphometric dimensions of the deltoid muscle in order to facilitate the revision of the intramuscular injection technique, resulting in greater success and less adversity.
The present work showed a bulky deltoid muscle, with a coarse texture, covering the dorsal, ventral and lateral parts of the shoulder joint and presenting three distinct bellies (anterior, lateral and posterior) with multipennate aspect. Moore et al. (2019) support these results, but describe the anterior and posterior parts as semipennate, and the lateral part as multipennate. The distal fixation of the deltoid muscle at deltoid tuberosity as observed in the present study is in agreement with that found by Moore et al. (2019) and Standring (2015).
Regarding the irrigation and innervation of the deltoid muscle, the same pattern as described by Standring (2015) was found, with participation of the acromial and deltoid branches of the thoracoacromial artery, the anterior and posterior circumflex humeral arteries, the subscapular artery and the deltoid branch of the profunda brachii artery, and the axillary nerve. However, other authors have proposed the irrigation only through the deltoid branch of the thoracoacromial artery and the posterior circumflex humeral artery (Moore et al., 2019).
Although variations and anomalies of the deltoid muscle are not common, Kayikcioglu et al. (1993) reported a bilateral separation of posterior fibers by a fascia that has not been previously described. The continuation of the deltoid muscle fibers in the trapezius muscle, the fusion with the pectoralis major muscle and the presence of additional slips in the vertebral margin of the scapula, infraspinatus fascia and the axillary margin of the scapula are the commonly reported variations of the deltoid muscle (Standring, 2015). In the present study, there was no variation in the deltoid muscle in relation to its morphology.
The morphometric description of the deltoid region has seldom been investigated. In this research, the descriptive analysis showed that the posterior margin length of the deltoid muscle represented the majority of the upper limb length and the smallest portion was attributed to the lateral margin length, while the anterior one showed an intermediate value. These dimensions can be explained anatomically by the points of proximal fixation of the deltoid muscle, that is, in the anterior margin and superior surface of the lateral third of the clavicle (AML), lateral margin and adjacent superior surface of the acromion (LML) and lower edge of the scapular spine crest (PML), as described elsewhere (Moore et al., 2019; Standring, 2015; Gardner et al., 1988).
Considering the mean values of deltoid belly thickness, it was found that the posterior one represented the most part of the belly width, suggesting that this analysis is related to the action of the deltoid muscle and the specific functions of the clavicular (anterior), acromial (lateral) and scapular (posterior) fibers. According to Standring (2015), the posterior fibers act with the latissimus dorsi and teres major muscles when pulling the arm back and turning it laterally, which seem to be intense and more frequent movements, in comparison to the anterior fibers that assist the pectoralis major muscle to pull the arm forward and rotate it medially, while the acromial fibers, aided by the supraspinatus muscle, abduct the arm until the joint capsule is tensioned.
The mean value (2.85 cm) of the distance from the neurovascular bundle to the intramuscular injection point (NBII) towards the posterior part, and often in the posterolateral part of the deltoid muscle, indicates that the neurovascular bundle is located within the region of application of the intramuscular injection. According to Kozier et al. (1993) and Rodger and King (2000), this region represents an imaginary triangle, whose base is an imaginary horizontal line of two to three finger widths, 2 to 5 cm below the lower margin of the acromial process, and the apex is formed inverted at the midpoint of the arm lateral face in line with the axilla.
Next, the morphometric parameters of the muscle length and belly thickness regarding the anterior, lateral and posterior parts of the muscle deltoid were compared. The significantly lower mean value of the lateral margin length compared to anterior and posterior ones can be explained by anatomical muscle format, which is a curved triangle in shape of the Greek letter delta (Testut and Latarjet, 1979; Standring, 2015) with the shortest length between the proximal and distal fixation located laterally, and the longest lengths in the anterior and posterior parts of the muscle. On the other hand, the mean value found for the anterior belly thickness, which was significantly lower than posterior one, suggests that the anterior part of the deltoid muscle is less functionally required compared to the posterior one, while the lateral part acts equal to the anterior and posterior ones. This analysis implies that the ideal place for the application of intramuscular injection should be the posterior part, as it presents greater thickness; however, the neurovascular bundle was found in this region. Therefore, it is not recommended to consider only the muscle thickness parameter to choose the best site for the intramuscular injection.
When comparing the mean values of all analyzed parameters between the right and left antimeres, the significant difference found only for the anterior belly thickness, with a higher value on the right antimere, can be explained by the fact that a large part of the world population is right-handed and, therefore, making more use of the right upper limb for routine tasks. Thus, it can be inferred that when intramuscular injections are administered in the anterior belly of the deltoid muscle, the most appropriate side would be on the right antimere, as it is thicker, while for injection in the other muscle bellies, there is no preference on the side.
In this research, possible correlations between some parameters were also examined. The positive correlation found between upper limb length and deltoid lateral margin length can be due to the fact that these measures are on the same axis, namely, longitudinal axis. Other positive correlations verified between the anterior belly thickness vs the belly width or the distance from neurovascular bundle and intramuscular injection point indicate that the anterior part of the deltoid muscle could be chosen as a possible site for injection administration, despite its smaller thickness.
Taken together, the results of the present study provide an anatomical basis to confirm that, in 100% of the analyzed pieces, the neurovascular bundle is included in the recommended area for intramuscular injection in the deltoid region as previously reported (Kozier et al., 1993; Rodger and King, 2000). McGarvey and Hooper (2005) proposed that the injection site be into the center of this area; however, this region would not be the most suitable for such procedure, as it presents an imminent risk of injury to neurovascular structures.
Other studies have also shown that the deltoid region may not be ideal for administering intramuscular injection. During the practice of professionals from the nursing team of a Teaching Hospital at surgical and gynecological units, where the deltoid region is one of the most chosen regions, it was observed that 78.12% of professionals delimited it incorrectly (Godoy et al., 2003). The authors state that the possibility of improper puncture of a vessel is great in this region, due to the presence of circumflex vessels that can be reached in the case of the incorrect location of the application site. Also, another study identified a variation in the anatomical location of the axillary nerve in the deltoid region (Meirelles and Filho, 2004). Considering the potential of the sequel caused by the neural injury, the authors indicate that the intramuscular injection in the deltoid muscle should not be the site of first choice. In addition, the risk of complications with the use of this route is much greater when compared to others, such as the superolateral quadrant of the gluteal region or the lateral face of the quadriceps muscle (Meirelles and Filho, 2004).
Finally, even though the action of drugs on the deltoid muscle was not herein verified, studies have shown that during intramuscular injection procedures, inflammatory lesions of the local soft tissues derive from the caustic action of the product, its concentration, volume and, more rarely, from the mechanical action of the needle, although the aggression to nerve endings has a similar origin. The oily drug, when penetrating the artery, generates emboli and occludes the lumen; thus, the medication in an aqueous vehicle, when gaining the lumen from the arterial and/or venous vessel, injures the endothelium and causes secondary thrombosis. Lesions of somatic and/or peri-vessels nerve endings trigger vasomotor sympathetic reflexes with vascular constriction and worsening of ischemia and/or infarction of tissues irrigated by these vessels (Duque and Chagas, 2009).
Limitations
This study was performed on formalin-fixed cadaveric upper limbs and, therefore, the effect of formalin on skin cannot be ruled out; thus, thickness of the subcutaneous tissue could not be measured and this may have influenced the morphometric parameters.
Furthermore, the body weights and sexes of most cadavers were unknown, making it impossible to estimate the fat layer in them, leading to generalized results.
Conclusions
The deltoid muscle consists of three parts (anterior, lateral and posterior); the anterior part has the smallest thickness and the lateral part, the smallest length. There is no morphometric difference between the antimeres, except for the thickness of the anterior belly with the highest value on the right side. The neurovascular bundle is located in the posterior and posterolateral part of the muscle and, therefore, within the area of intramuscular injection. Thus, considering the morphology and morphometric measurements of the deltoid muscle, the most anterior part of the lateral belly and the whole anterior belly seem to be more favorable sites for administering intramuscular injections.
ACKNOWLEDGEMENTS
We would like to thank the National Council for Scientific and Technological Development (Conselho Nacional de Desenvolvimento Científico e Tecnológico – CNPq, Brazil) for support this research.
Related articles
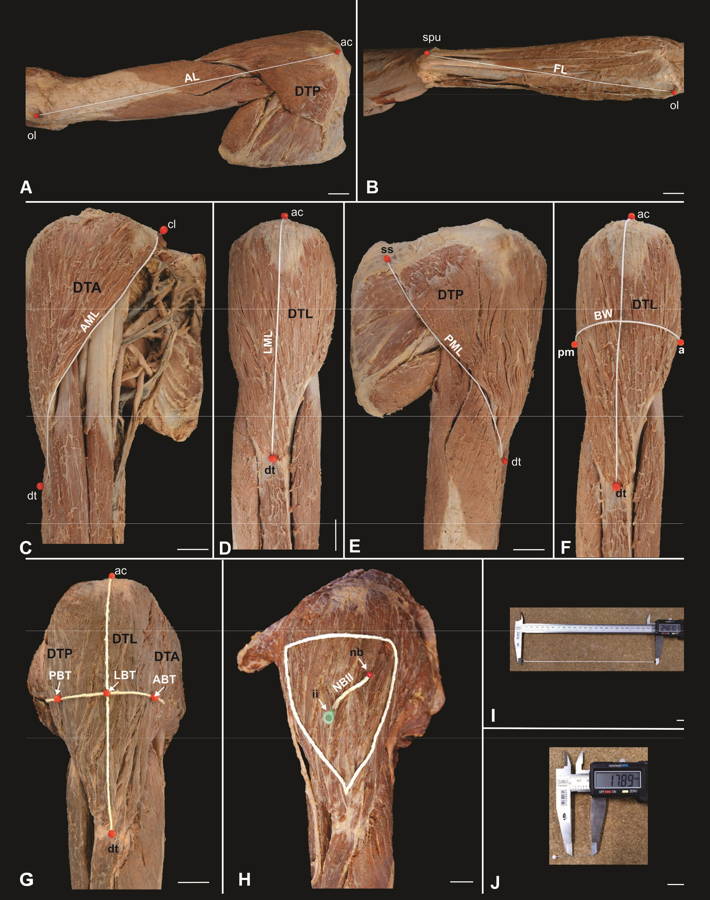 Fig. 1.- Upper limb and shoulder region showing the deltoid muscle parts (anterior part – DTA, lateral part – DTL, posterior part - DTP) and the reference points for the measurements of the analyzed parameters. A. arm length (AL), B. forearm length (FL), C. anterior margin length (AML), D. lateral margin length (LML), E. posterior margin length (PML), F. belly width (BW), G. anterior belly thickness (ABT), lateral belly thickness (LBT), posterior belly thickness (PBT), H. application area of the intramuscular injection and distance between the neurovascular bundle and the intramuscular injection point (NBII), I Caliper and string, J. Caliper and pins. ac: acromion, cl: clavicle, ss: scapula spine, nb: neurovascular bundle, ii: intramuscular injection, am: anterior margin, pm: posterior margin, ol: olecranon, spu: styloid process of the ulna, dt: deltoid tuberosity. Scale bar: A-H: 3 cm. I-J: 1 cm.
Fig. 1.- Upper limb and shoulder region showing the deltoid muscle parts (anterior part – DTA, lateral part – DTL, posterior part - DTP) and the reference points for the measurements of the analyzed parameters. A. arm length (AL), B. forearm length (FL), C. anterior margin length (AML), D. lateral margin length (LML), E. posterior margin length (PML), F. belly width (BW), G. anterior belly thickness (ABT), lateral belly thickness (LBT), posterior belly thickness (PBT), H. application area of the intramuscular injection and distance between the neurovascular bundle and the intramuscular injection point (NBII), I Caliper and string, J. Caliper and pins. ac: acromion, cl: clavicle, ss: scapula spine, nb: neurovascular bundle, ii: intramuscular injection, am: anterior margin, pm: posterior margin, ol: olecranon, spu: styloid process of the ulna, dt: deltoid tuberosity. Scale bar: A-H: 3 cm. I-J: 1 cm.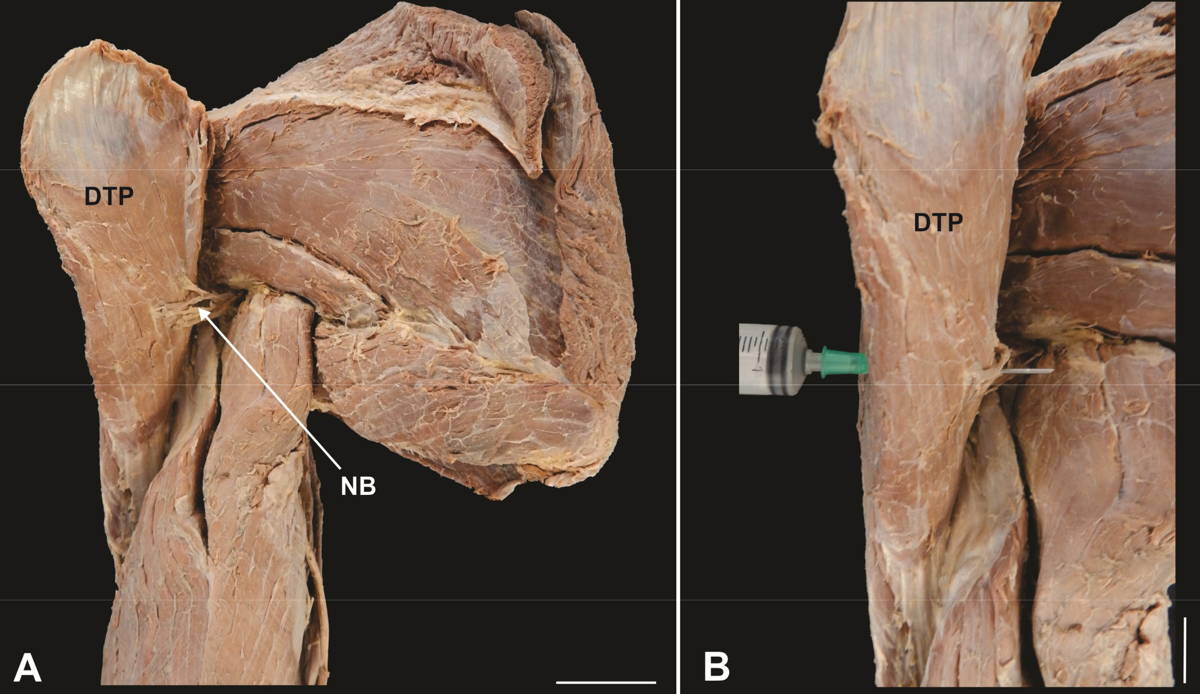 Fig. 2.- Posterior view of the upper limb and shoulder region. A. Posterior belly of the folded deltoid muscle (DTP) showing the neurovascular bundle (NB), B. Needle through the posterior belly of the deltoid muscle. Scale bar: 3 cm.
Fig. 2.- Posterior view of the upper limb and shoulder region. A. Posterior belly of the folded deltoid muscle (DTP) showing the neurovascular bundle (NB), B. Needle through the posterior belly of the deltoid muscle. Scale bar: 3 cm.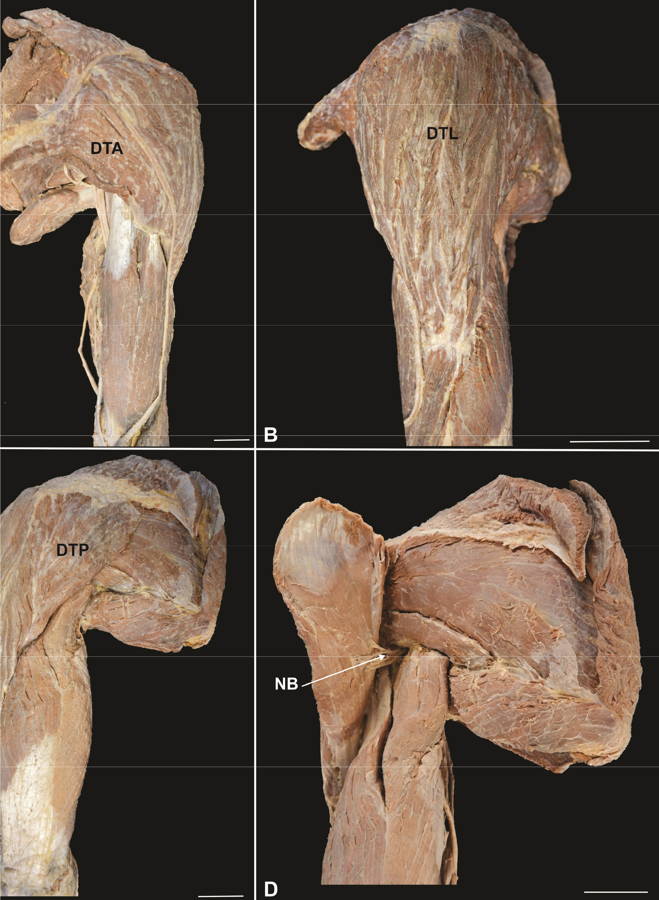 Fig. 3.- Left upper limb showing the deltoid muscle. A. Anterior view, B. Lateral view, C. Posterior view, D. Posterior view - folded deltoid muscle. DTA: Anterior or clavicular part, DTL: Lateral or acromial part, DTP: Posterior or spinal part, NB: Neurovascular bundle. Scale bar: 3 cm.
Fig. 3.- Left upper limb showing the deltoid muscle. A. Anterior view, B. Lateral view, C. Posterior view, D. Posterior view - folded deltoid muscle. DTA: Anterior or clavicular part, DTL: Lateral or acromial part, DTP: Posterior or spinal part, NB: Neurovascular bundle. Scale bar: 3 cm. Fig. 4.- Comparison of the mean values (cm) of the length (A) and thickness (B) morphometric parameters of the deltoid muscle. AML: anterior margin length, LML: lateral margin length, PML: posterior margin length, ABT: anterior belly thickness, LBT: lateral belly thickness, PBT: posterior belly thickness. ** p < 0.01; *** p < 0.001. (Friedman test and Dunn’s multiple comparison post-test).
Fig. 4.- Comparison of the mean values (cm) of the length (A) and thickness (B) morphometric parameters of the deltoid muscle. AML: anterior margin length, LML: lateral margin length, PML: posterior margin length, ABT: anterior belly thickness, LBT: lateral belly thickness, PBT: posterior belly thickness. ** p < 0.01; *** p < 0.001. (Friedman test and Dunn’s multiple comparison post-test).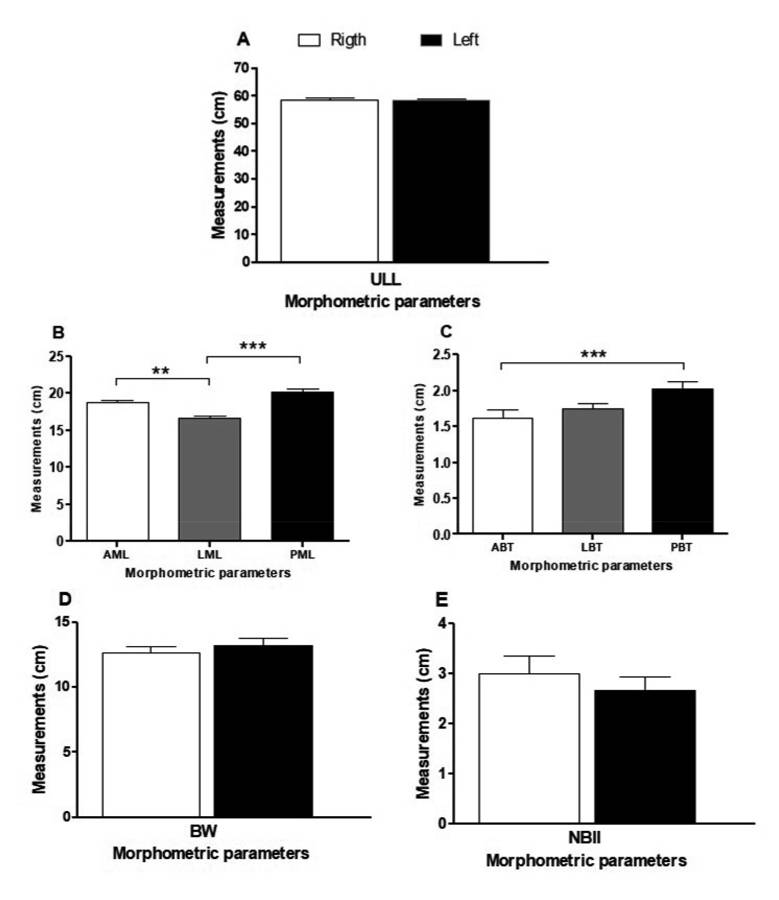 Fig. 5.- Comparison of the mean values (cm) of the morphometric parameters obtained from the upper limb and the deltoid muscle between the right and left antimeres. A. ULL: upper limb length, B. AML: anterior margin length, LML: lateral margin length, PML: posterior margin length, C. ABT: anterior belly thickness, LBT: lateral belly thickness, PBT: posterior belly thickness, D. BW: belly width, E. NBII: distance from the neurovascular bundle to the intramuscular injection point. * p = 0.0433 (Student t test).
Fig. 5.- Comparison of the mean values (cm) of the morphometric parameters obtained from the upper limb and the deltoid muscle between the right and left antimeres. A. ULL: upper limb length, B. AML: anterior margin length, LML: lateral margin length, PML: posterior margin length, C. ABT: anterior belly thickness, LBT: lateral belly thickness, PBT: posterior belly thickness, D. BW: belly width, E. NBII: distance from the neurovascular bundle to the intramuscular injection point. * p = 0.0433 (Student t test).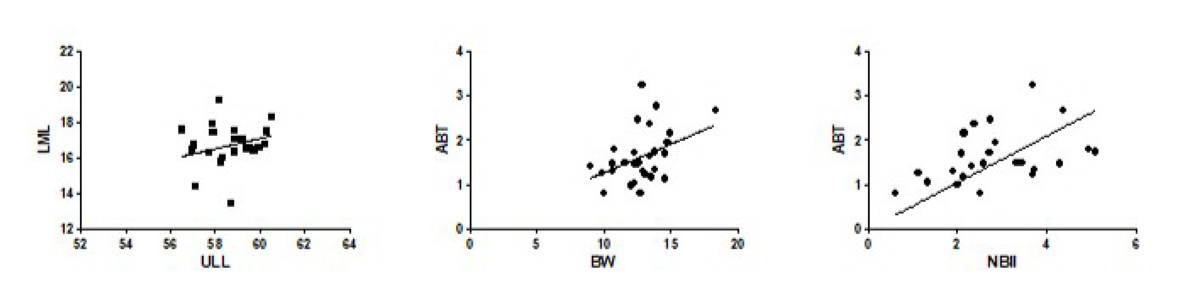 Fig. 6.- Dispersion curve and correlation between the morphometric parameters obtained from the upper limb and the deltoid muscle. ULL: upper limb length, LML: lateral margin length, BW: belly width, ABT: anterior belly thickness, NBII: distance from the neurovascular bundle to the intramuscular injection point. (Pearson or Spearman correlation test).
Fig. 6.- Dispersion curve and correlation between the morphometric parameters obtained from the upper limb and the deltoid muscle. ULL: upper limb length, LML: lateral margin length, BW: belly width, ABT: anterior belly thickness, NBII: distance from the neurovascular bundle to the intramuscular injection point. (Pearson or Spearman correlation test).BEECROFT PC, REDICK S (1989) Possible complications of intramuscular injections on the pediatric unit. Pediatr Nurs, 15: 333-336.
CASSIANI SHB, RANGEL SM, TIAGO F (1998) Complicações após aplicações, por via intramuscular, do diclofenaco de sódio: estudo de um caso. Medicina (Ribeirão Preto), 31: 99-105. https://doi.org/10.11606/issn.2176-7262.v31i1p99-105
CASTELLANOS BEP (1977) Estudo sobre as regiões para aplicação de injeção por via intramuscular. Rev Esc Enferm USP, 11: 261-324. https://doi.org/10.1590/0080-6234197701100300261.
COCOMAN A, MURRAY J (2008) Intramuscular injections: a review of best practice for mental health nurses. J Psychiatr Ment Health Nurs, 15: 424-434.
COSKUN H, KILIC C, SENTURE C (2016) The evaluation of dorsogluteal and ventrogluteal injection sites: a cadaver study. J Clin Nurs, 25: 1112-1119.
CRAVEN RF, HIRNLE CJ (2008) Fundamentals of nursing: human health and function, 6th ed. Lippincott Williams and Wilkins, Philadelphia.
DA SILVA PS, VAZ VIDAL S (2013) Las relaciones anatómicas involucradas en la administración de medicamentos por vía intramuscular: un campo de estudio de la enfermera. Enferm Glob, 12: 170-182. https://doi.org/10.6018/eglobal.12.2.143231
DUQUE FLV, CHAGAS CAA (2009) Acidente por injeção medicamentosa no músculo deltoide: lesões locais e à distância, revisão de 32 casos. J Vas Bras, 8: 238-246. https://doi.org/10.1590/S1677-54492009000300009
GARDNER E, GRAY DJ, O’RAHILLY R (1988) Anatomia: estudo regional do corpo humano. 4th ed. Guanabara Koogan, Rio de Janeiro.
GIOVANI AMM (2006) Calculation and administration of drugs. 10th ed. Scrinium, São Paulo.
GODOY S, NOGUEIRA MS, MENDES IAC (2004) Intramuscular medication application: knowledge analysis among nursing professionals. Rev Esc Enferm USP, 38: 135-142. https://doi.org/10.1590/s0080-62342004000200003
GREENBLATT DJ, ALLEN MD (1978) Intramuscular Injection - site complications. JAMA, 240: 542-544.
HOPKINS U, ARIAS CY (2013) Large-volume IM injections: a review of best practices. Oncol Nurse Advisor, 4: 32-37.
KAYIKÇIOGLU A, CELIK HH, YILMAZ E (1993) An anatomic variation of the deltoid muscle (case report). Bull Assoc Anat (Nancy), 77: 15-16.
KEEN MF (1986) Comparison of intramuscular injection techniques to reduce site discomfort and lesions. Nurs Res, 35: 207-210.
KOZIER B, ERB G, BLAIS K (1993) Techniques in clinical nursing. 4th ed. Prentice Hall, London.
LOSEK JD, GYURO J (1992) Pediatric intramuscular injections: do you know the procedure and complications? Pediatr Emerg Care, 8: 79-81.
MANDARIM-LACERDA CA (1995) Métodos quantitativos em morfologia. UERJ, Rio de Janeiro.
MCGARVEY MA, HOOPER ACB (2005) The deltoid intramuscular site in the adult. Current practice among general practitioners and practice nurses. Ir Med J, 98: 105-107.
MCIVOR A, PALUZZIM M, MEGUID MM (1991) Intramuscular injection abscess: past lessons relearned. N Engl J Med, 324: 1897-1898.
MEIRELES H, MOTTA-FILHO GR (2004) Lesão do nervo axilar causada pela injeção intramuscular no deltóide: relato de caso. Rev Bras Ortop, 39: 615-619.
MOORE KL, DALLEY AF, AGUR AMR (2019) Anatomia orientada para a clínica. 8th ed. Guanabara Koogan, Rio de Janeiro.
NICOLL LH, HESBY A (2002) Intramuscular injection: an integrative research review and guideline for evidence-based practice. Appl Nurs Res, 15: 149-162.
RODGER MA, KING L (2000) Drawing up and administering intramuscular injections: a review of the literature. J Adv Nurs, 31: 574-582.
RODRIGUES H (2010) Técnicas anatômicas. 4th ed. Edson Maltez Heringer, Vitória.
SMALL SP (2004) Preventing sciatic nerve injury from intramuscular injections: literature review. J Adv Nurs, 47: 287-296.
STANDRING S, editor (2015) Gray’s Anatomy: the anatomical basis of clinical practice. 41th ed. Elsevier, Amsterdam.
TESTUT L, LATARJET A (1979) Tratado de anatomia humana. 9th ed. Publishing Company Salvat, Barcelona.
THOMAZ JB, BALTAR CAF (1988) Acidente isquêmico no membro superior produzido por injeção intramuscular de penicilina benzatina. Arq Bras Med, 62: 175-178.
VALDERRAMA JAF, MIGUEL RE (1981) Fibrosis of the gluteus maximus: a cause of limited and adduction of the hep in children. Clin Orthop Relat Res, 156: 67-78.