INTRODUCTION
Phoenix dactylifera L. (date palm) fruit, due to its rich components, has been implicated to have a wide range of beneficial medicinal properties such as immunostimulant, hepatoprotective, nephroprotective, free radical scavenging, antimutagenic, antimicrobial, anticancer and antioxidant properties (Al-Farsi and Lee, 2008; Ishurd et al., 2004; Vayalil, 2002). Phoenix dactylifera L. is highly rich in dietary fiber, fat, carbohydrate, vitamins and amino acids (Zhang et al., 2013; Ali and Abdu, 2011). The antioxidant efficacies of Phoenix dactylifera L. are attributed to a wide range of flavonoids, phenolic compounds, sterols and volatile oil (Baliga et al., 2011; Al-Farsi and Lee, 2008; Hong et al., 2006).
The usage and cultivation of Phoenix dactylifera L. plant dates back to about 5000 years. The plant belongs to the family of Arecaceae; it is a diploid, perennial, monocotyledonous and dioecious plant (Ragab et al., 2013). In 2018, Mobasher and other scientists reported that both the aqueous and methanolic extract of Phoenix dactylifera L. significantly ameliorate Azithromycin-induced hepatic and renal toxicities; the aqueous extract of the plant is more potent and active than the methanolic extract of Phoenix dactylifera L., according to the data from this research work (Mobasher et al., 2018).
Gossypol is a lipid-soluble polyphenolic compound extracted from the cotton plant (genus Gossypium) and the tropical tree Thespesia populnea (L.) Sol. ex Correa (Hoda and Bahram, 2014). Gossypol, due to it phenolic component, may possess beneficial effects to the organism, but when abused it may result in debilitating effects. The gossypol LD50 has been determined for several mammalian species, including rats (925-1,350 mg/kg), mice (500-950 mg/kg), rabbits (350-600 mg/kg), guinea pigs (280-300 mg/kg) (Eagle et al., 1948), and pigs (550 mg/kg) (Lyman et al., 1963).
Gossypol have been reported to possess varying beneficial properties in humans and animals due to its chemical composition such as anti-oxidative (Wang et al., 2008), anti-tumor (Zhai at el., 2006), anti-viral (Vander Jagt et al., 2000; Lin et al., 1993), anti-malaria (Razakantoanina et al., 2000; Royer et al., 1986), antimicrobial (Vadehra et al., 1985) and reduced cholesterol production (Shandilya et al., 1982) properties.
In contrast to all the aforementioned beneficial properties, Gossypol toxicities have been also explored and documented. Gossypol has been found out to have antifertility properties, without altering hormonal production (i.e., non-steroidal) and expression in male. Instead, it acts as a contraceptive by subduing enzyme production responsible for energy metabolism in sperm and spermatogenic cells in humans and animals alike (Coutinho, 2002; Wang et al., 2009). Amy and others reported that gossypol, administered in increasing dosage to Northern bobwhites, resulted in structural alterations in certain tissues such as hepatocellular pigmentation, pancreatic necrosis and intestinal toxicity (Amy et al., 2019). Gossypol may also possess immunosuppressive properties, as reports have shown that gossypol cause a reduced number of leukocytes and primary lymphocytes, which affects the animals’ immunocompetence (Braga et al., 2012). In vivo and in vitro mouse experiments also depicted gossypol’s immunosuppressive tendencies by inhibiting lymphocyte proliferation and inducing apoptosis (Xu et al., 2009).
This research work aimed to elucidate the toxic effects, if any, of gossypol on liver tissue in murines, as well as the possible protective actions of Phoenix dactylifera L.
MATERIALS AND METHODS
Animal source and handling
A total of thirty (35) adult Wistar rats weighing between 150-200g were procured from the Animal Holding Facility in Jos, for this experiment. Animals were maintained in a controlled environment (22 ± 2C; 14 h of light from 06:00 h to 20:00 h) and had free access to pellet laboratory chow rat-mouse diet and acclimatized for four weeks before the experiment commenced. The rats were fed with standard diet (growers mash); water was given ad libitium and maintained under standard conditions. The animal room was kept at 25-27 degrees Celsius under day and night. The rats were distributed into seven (7) groups of five (5) rats each.
Ethical clearance
Animals were treated humanely and with regard to alleviation of suffering for the care and use of laboratory animals. The experimental protocol was approved by the Ethical Review Committee (ERC) of Bingham University, Karu, Nigeria. The study was carried out in the animal house of the College of Health Sciences, Bingham University, Karu, Nigeria.
Extract preparation
Cotton seed was obtained from Dengi market, Plateau state, Nigeria. The seeds were pounded to powder using a mortar and pestle and the cotton seed oil was extracted using the solvent extraction method described by Orheva and Efomah (2012). The experimental process involved the following; collection of seeds, cleaning of seeds, drying, cooling, size reduction, weighing of the crushed seeds, solvent extraction, weighing of the cottonseed cake, recovery of solvent and recovery of crude cottonseed oil. The samples collected were properly cleaned in order to remove any foreign materials. They were oven-dried in the laboratory at a temperature of 130°C, to a moisture content of 12%. This was done because the lesser the moisture content, the more the oil yields (Taiwo et al., 2008). The seeds were then crushed into powder using Thomas Willey mill (Model ED-5). Twelve (12) grammes (g) of the crushed sample was weighed and mixed with 5 ml of N-hexane. The mixed sample was placed on a filter paper and the filter paper was then properly folded and inserted into the assembled soxhlet apparatus. The weight of the filter paper and sample was recorded. One hundred and fifty milliliters (150 ml) of the solvent (N- hexane) was measured using a measuring cylinder, and then poured into a five-hundred-milliliter (500 ml) round-bottom flask, which is the lower part of the Soxhlet apparatus. This was now heated with a heating mantle at 60oC for 6 hours. As the solvent boiled, it evaporated into the reflux condenser, and this hot solvent vapour was cooled by the surrounding water that flowed continuously through the soxhlet arrangement. The cooled solvent then condensed back into the portion of the soxhlet containing the folded sample and this facilitated the extraction of the oil from the sample. The extracted solution in the round-bottom flask was a combination of oil and solvent. The sample left, after the oil had been removed, was subjected to hot pressing using hydraulic press to remove the bulk of the oil remaining in the press cake (Orhevba and Efomah, 2012)
Gossypol was extracted from the cottonseed oil using 70% cold acetone. This is because the decomposition rate of gossypol is lower in acetone than in other organic solvents such as methanol, chloroform, ethanol, and acetonitrile (Nomeir and Abou-Dounia, 1982).
Date palm fruits were obtained from Masaka market in Nassarawa state. The seeds were removed from the fruit and the pulp was pounded to powder using mortar and pestle. 435 g of the powder was soaked in 4350 ml of distilled water for 24 hours. The solution was then filtered using fine sieve and the residue was discarded. The filtrate was evaporated at 60°C using water bath till a thick semisolid paste with a fruity smell was obtained. This was dissolved in phosphate buffer solution before being administered to the rats. An aqueous extract was selected because most of the antioxidant components in dates are extracted in water (Vayalil, 2002; Al-Farsi et al., 2005b).
Preparation of Phoenix dactylifera extract
The extraction procedure was adapted from the reports in Emilia et. al. (2018); date fruit pod was obtained and authenticated in the Biology Department, Bingham University, Nigeria. The date fruit pod was broken in order to remove the seeds and pounded. The powder was extracted twice with distilled water (1:10 w/d). 125g of powdered date fruit pod was weighed into 2x500 ml conical flask; 70 % ethanol was introduced and allowed over-night; the content was the shaken for an hour and filtered through a Whatman #43 filter paper; several paths of rinsing were done. The filtered solution was then concentrated by rotary evaporation. The extract was stored at 6°C overnight. The extract obtained from this procedure was subjected to further extraction by adding acetone water to remove the oil. The acetone water filtrate was again extracted by using chloroform; the aqueous layer was the concentrated to dryness to obtain the needed extract, which is the normal acetone water extract.
Animal grouping
The rats were distributed into seven (7) groups (n = 5). Group A received- 0.1ml of phosphate buffer solution; Group B received – 40 mg/kg of Phoenix dactylifera only; Group C received - 15 mg/kg gossypol; Group D received – 30 mg/kg of gossypol; Group E received - 15 mg/kg gossypol + 40 mg/kg Phoenix dactylifera; Group F received - 15 mg/kg Vitamin E; Group G - 15 mg/kg gossypol + 15 mg/kg vitamin E. The administration of experimental substances was done via oral gavage using oropharyngeal cannula for a period of 56 days.
Sample collection and processing
The animals were euthanized by cervical dislocation twenty-four hours after last administration and the liver tissue was excised. The left lobe of the liver from each rat was fixed in Bouin’s fluid for routine histological haematoxylin & eosin (H/E), and immunohistochemical (CK7) stains while the other was homogenized in 5% sucrose solution for enzyme assay.
Histological preparation
The histology of the liver was done by modification of the method described by Akpantah et al. (2003). The organs were cut in slabs of about 0.5cm thick transversely and fixed in 10% buffered formalin for a day, after which it was transferred to 70% alcohol for dehydration. The tissues were passed through 90% and absolute alcohol and xylene for different durations before they were transferred into two (2) changes of molten paraffin wax for 1hr each in an oven at 65°C for infiltration. They were subsequently embedded and serial sections were cut using rotary microtome at six microns. The tissues were transferred onto albuminised slides allowed to dry on a hot plate for 2 minutes. The slides were then dewaxed with xylene and passed through absolute alcohol 2 changes); 70% alcohol, 50% alcohol and then to water for 5 minutes. The slides were then stained with haematoxylin and eosin. The slides were mounted in Canada balsam. Photomicrographs were taken using.
Biochemical assay
Enzyme biochemistry
The homogenized liver tissue was utilized for malondaldehyde (MDA), glutathione (GSH), superoxide dismutase (SOD), glucose phosphate dehydrogenase (G6PDH) and lactate dehydrogenase (LDH) assay. The oxidative stress and bioenergetics markers were assessed by an enzyme-linked immunosorbent assay kit (IBL-America, Minneapolis, Minnesota, USA).
Excised liver tissues were put in a Lao style mortar containing 1ml of 0.25 Mn (5%) sucrose solutions and were homogenized thoroughly. Tissue homogenate was collected in 5 ml plain serum bottles for enzyme assay; Glucose-6-phosphate dehydrogenase (G6PD), superoxide dismutase (SOD), Malondialdehyde (MDA), Glutathione peroxidase (GSH-Px) and Lactate Dehydrogenase (LDH)
Determination of Malondialdehyde (MDA) level in tissue homogenates
Malondialdehyde level in tissues were measured according to the protocol outlined by Stocks and Domandy (1971), 0.1 ml of homogenate was pipette into a plastic test tube. 1 ml of 20% Trichloroacetate was added to it. The mixture was processed and centrifuged at 2000 g for 5 minutes. 0.5 ml of the supernatant was pipetted into a pyrex test tube 0.05 ml of 10.0 µmol/L of 1,1,3,3-Tetramethoxypropane was pipetted into another pyrex test tube (std). 0.5 ml of Trichroacetic acid solution and 1.0 ml of Thibarbituric acid was pipetted into a 3rd pyrex test tube (blank). All tubes were stoppered tightly. The test tubes were heated in a water bath at 100ºC for 20 minutes. All tubes were cooled in water. The spectrophotometer was blanked using the reagent blank at 532 nm. Absorbance of test and standards were read.
Determination of Glucose 6 phosphate Dehydrogenase (G-6-PDH) Activity in tissue homogenate
Activity of G-6-PDH in homogenate was measured using the method of Lohr and Walker (1974). A reaction mix of 1350 µl TRAP buffer (triethanolamine 50 mM, ethylenediaminetetraacetic acid 5 mM pH 7.5), 50 µl of magnesium chloride 1M, 25 µl of haemolysate (0.9% w/v sodium chloride saline washed erythrocytes disrupted with a saturated solution of digitonine), 25 µl of NADP (nicotinamide-adenine-dinucleotide phosphate 30mM) were started by finally adding 25 µl of G6P (glucose 6 phosphate, 50mM). Photometric extinction rate compared to a blank without haemolysate was recorded at 37°C. Results of the G6PD testing values were calculated in units per µMol metabolic rate/1011 erythrocytes/min (normal value: 30.5 ± 4.5). Activity was then converted into Units per g Haemoglobin (U/g Hb, Trinity Biotech for 37°C at 366nn, normal range 4.6-13.5 U/g Hb) by the following formula: (activity in μmol metabolic rate/1011 erythrocytes/min * RBC M/µL * 0.66)/Hb g/dL.
Determination of Lactase Dehydrogenase (LDH) Activity in tissue homogenate
Activity of LDH in the homogenate was measured according to the method in Jeyaraman et al. (2009). The homogenate was centrifuged at 10,000×g for 10 minutes at 4ºC. The clear supernatant obtained was used for the measurement of LDH activity.
Reagents
0.2 M Tris⋅HCl, pH 7.3
6.6 mM NADH in above 0.2 M Tris⋅HCl buffer, pH 7.3
30 mM Sodium pyruvate in above 0.2 Tris⋅HCl buffer, pH 7.3
Enzyme
Dissolve at 1 mg/ml in 0.2 M Tris⋅HCl buffer. Dilute enzyme prior to use to obtain a rate of 0.02-0.04 ΔA/min. in Tris buffer and keep cold.
Procedure
Spectrophotometer was set at 340 nm and 25°C.
Pipette into cuvette as follows:
|
Tris⋅HCl, 0.2 M pH 7.3
|
2.8 ml
|
|
6.6 mM NADH
|
0.1 ml
|
|
30 mM Sodium pyruvate
|
0.1 ml
|
Incubate was done spectrophotometer 4-5 minutes to achieve temperature equilibration and establish a blank rate.
Add 0.1 ml of appropriately diluted enzyme and record ΔA340/min from initial linear portion.
Calculation
ΔA340/min
Unit/ml = _________________________________
6.22Xmg enzyme/ml reaction mixture
Determination of Superoxide dismutase (SOD)
Superoxide dismutase activity in homogenates was determined using the method of Misra and Fridovich, (1972). 200 μl R1 reagent (WTS solution diluted 20-fold with buffer) was pipetted into a plastic cuvette and incubated at 37°C for 108 sec. Subsequently, 20 μl of sample was added and in 378 sec. The reaction was started by adding 20 μl R2 reagent (enzyme solution diluted 167-fold with buffer). The reaction was incubated for 72 sec and then absorbance was measured at λ=450 nm. The kinetic reaction was measured for 108 sec and the absorbance was recorded every 9 sec.
Determination of Malondialdehyde (MDA)
Malondialdehyde (MDA) activity in homogenates was determined using the method by Reilly and Aust (1999) as shown below;
Reagents
30% Tricarboxylic Acid (TCA): 3g of TCA was added to 10ml of distilled water.
0.75% Thiobarbituric Acid (TBA): 0.225g of Thiobarbituric Acid (TBA) was added to 30ml of 0.1M of HCl.
0.15M Tris KCl buffer solution (pH 7.4)
1.12g of KCl was added to 2.36g of Tris base.
200ml of distilled water was added
pH was adjusted to 7.4
Procedure
0.4ml of sample was added to (1.6ml of Tris- KCL buffer and 0.5ml of 30% TCA).
0.5ml of TBA was added.
The solution was incubated for 45minutes at 80˚C (produced a pink colour).
Then cooled in ice and centrifuged at 14000g for 15minutes.
The absorbance was read at 532nm.
Calculation
Absorbance x Volume of Mixture
MDA
(Unit/mg protein) = __________________________________
E532 x Volume of Sample X mg protein
Where E532 = Molar absorptivity at 532nm = 1.56 x 105
Data analysis
Data collected were analysed using one-way analysis of variance (ANOVA) followed by Tukey’s (HSD) multiple comparison test with the aid of GraphPad Prism v.6 (GraphPad Software, Inc., La Jolla, CA, USA). Data were presented as means ± SEM (standard error of mean). P value less than 0.05 (P < 0.05) was considered statistically significant.
RESULTS
Histological observations
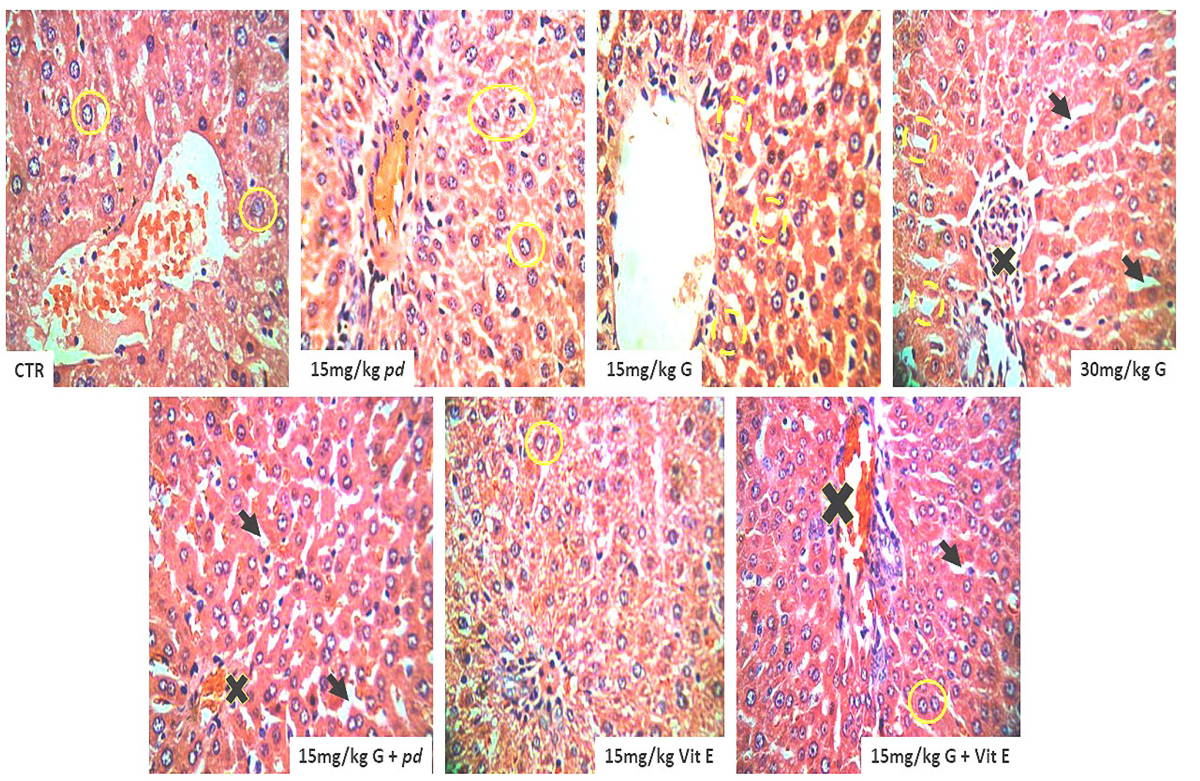
Administration of Phoenix dactylifera L. and Vitamin E preserved the hepatic histo-architecture that characterized the histological features of the liver in the control animals that received only phosphate buffered saline (PBS). The integrity of the hepatic sinusoids and the arrangement of the horizontal placed hepatic plate with the intact nuclei were maintained. Continuous cell division as indicated in ure 1 among the positive and the negative control animals was significant by the karyolysis of the nucleus. However, administration of the gossypol present in cottonseed extracts causes derangement in the horizontal hepatic plates. Degeneration in the hepatic nuclei was a result of chromatolysis and pkynosis. Increase in vacuolation and loss of hepatic sinusoidal spaces was evident in the gossypol-treated animals as shown in Fig. 1 Engorged blood sinusoid, rupture, granular degeneration of hepatocytes, oedema, focal necrosis, and proliferation of fibroblasts were the alterations. Co-administration of gossypol with Phoenix dactylifera L. and Vitamin E shows hepatopretective impacts of Phoenix dactylifera L. and Vitamin E. The testicular histology was preserved similar to the control animals and the arrangement in the hepatic sinusoid with the intact nuclei demonstrate the protective effects of both the Vitamin E and the Phoenix dactylifera L. ethanolic extracts.
Cytokeratin 7 (CK7) demonstration has been shown to be generally negative in hepatocellular tumour and in colorectal carcinoma, Merkel cell carcinoma, prostatic adenocarcinoma, adrenocortical tumours and squamous cell carcinoma. The hepatic demonstration of Cytokeratin 7 (CK7) in the gossypol treated groups showed less affinity for the Cytokeratin 7 (CK7) protein demonstrations (negative) particularly in the group that was given 30 mg/g body weight of cotton seed extracts. Cytokeratin 7 (CK7) is the marker expression in membranous and cytoplasmic normal epithelia; the control animal significantly expressed this protein in line with the animals that were given Phoenix dactylifera L. and Vitamin E when compared with the level of expression in gossypol treated animals as shown in Fig. 2. The expression of Cytokeratin 7 (CK7) in the negative control posed with less variation with the positive control group, with moderate expression of Cytokeratin 7 (CK7) among the groups co-treated with the gossypol and Phoenix dactylifera L as well as those given Vitamin E.
It is worth note that macrosteatosis and microsteatosis were most prominent histopathologic features among the gossypol treated group particularly the group with 30 mg/g body weight of cotton seed extracts. The lobular fat deposition demonstrating the macrosteatosis was more defined in the periportal, midzonal, or centrilobular regions of the hepatic lobule. Vitamin E treated group display a unique pattern of macrosteatosis and microsteatosis across the periportal, midzonal, or centrilobular regions of the hepatic lobule, however in a consistent and moderate patterns.
Oxidative stress & Cellular bioenergetics markers assay
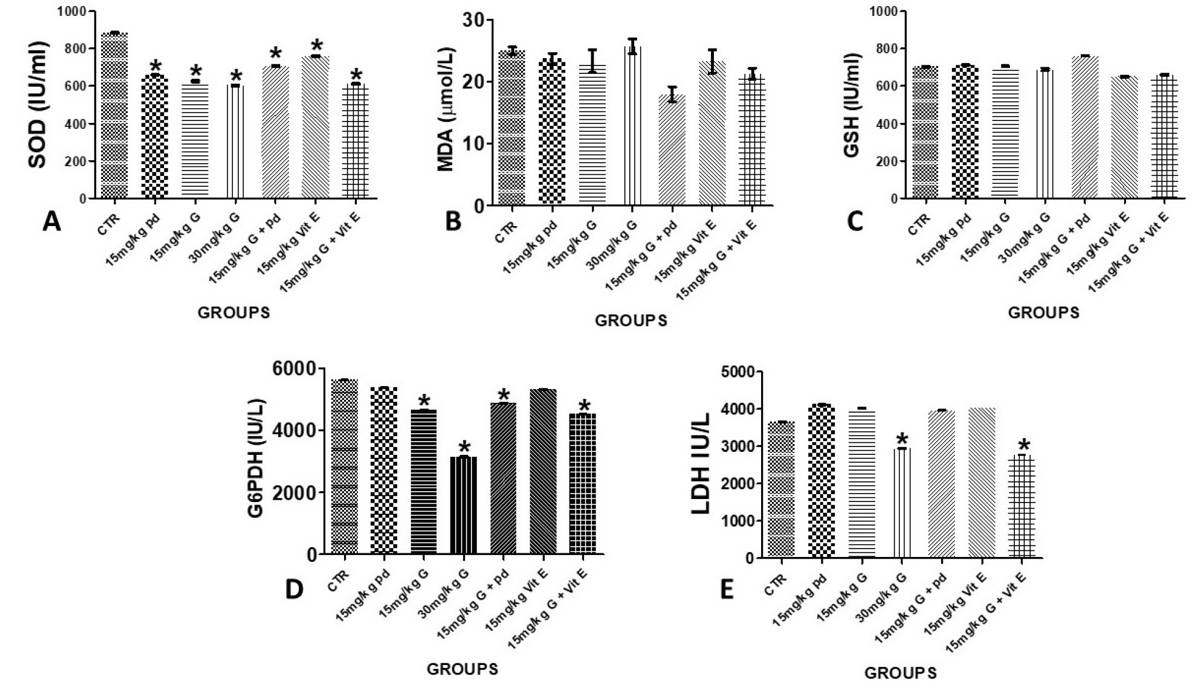
The levels of glutathione metabolizing enzymes, G6PD and LDH was measured as well, and decrease in the level of G6PD and LDH activities was noted as shown in figure 3 among the animals treated with cotton seed extract, gossypol. This decrease could normally result from the deficient flow of G-6-P through the hexose monophosphate shunt and decreased supply of reduced NADPH for the conversion of GSSG to GSH, thereby causing a switch in the NADP+/NADPH ratio in favour of NADP. However, across the positive and the negative control groups the enzymes’ integrity was preserved and the activities of these enzymes were maintained. The intervention groups, gossypol damage liver treated with Phoenix dactylifera L. and Vitamin E significantly maintained the carbohydrate enzymes’ activities similar to the control animals as revealed in Fig. 3.
DISCUSSION
Oxidative stress has been considered as a conjoint pathological mechanism with the attendant liver (hepatic) injury. Reactive oxidative substances such as; alcohol, drugs, environmental pollutants and irradiation, induce oxidative stress in liver tissue. This study, therefore investigated structural alterations, and the changes in the level of activities of the antioxidant enzymes and the enzymes of carbohydrates metabolism in animals exposed to varying dosage of cotton seed extract (Gossypol) and the hepato-protective effects of Phoenix dactylifera and vitamin E was examined as well. Saber and Shalaby (2012) noted impaired hepatic lobules and derangement of the hepatic sinusoid as well as enlargement and congestion of blood vessel and marked cytoplasmic vacuolization among the pregnant mice treated with oral metiram, acting fungicide of dithiocarbamate group. These findings agreed with our histological observations using H/E stains as specified in Fig. 1 above; the gossypol treatment significantly disrupt the horizontal plate arrangement of hepatocytes, increased cytoplasmic vacuolation as a result of hepatocytes loss and glandular tissue damage. Our study revealed that most cells of the gossypol treated animals showed nuclei with signs of karyolysis and pyknosis as well as obvious fatty degeneration indicated by large number of fatty droplets with different size was observed in accordance with the study conducted by Brante et al. (2010).
He observed macrosteatosis and microsteatosis characterised the histopathologic of animals treated with alcohol intoxication, with most animals displaying panlobular or defined foci of microsteatosis in periportal, midzonal, or centrilobular regions of the hepatic lobule. Therefore, gossypol in this study had profound histological alteration in the hepatic tissue similar to the damage caused by known free-radical-generating substances such as the alcohol administration. Gossypol hepatotoxic, ascites and hepatocyte degeneration (strong cytoplasmic eosinophilia and nuclear pyknosis) had been observed in rats that received a single intraperitoneal gossypol dose of 25 mg/kg BW or 30 mg/kg BW, with characteristic mitochondrial vacuolation, an enlarged endoplasmatic reticulum, an expanded perinuclear space, and collagen fiber proliferation in the perisinusoidal space that is according to Lordelo (2005).
Administration of Phoenix dactylifera extracts as a natural ascorbic acid substance, with well documented antioxidant capability according to Brady (2011), provides protective mechanism for the gossypol hepatic damage. Phoenix dactylifera extracts has been proven to have antioxidants that prevent oxidative stress related to diseases such as cancer, aging, inflammation and cardiovascular diseases by eliminate free radicals which contribute to theses chronic diseases (Blomhoff et al., 2010). Histological appearances of the liver treated with Phoenix dactylifera extracts and vitamin E was maintained as observed in this study. This finding could be explained along Al-Qarawi et al. (2003) work that showed that date palm fruit had both protection and restoration effects of liver damage in the rats.
Cytokeratin 7 (CK7) has been proven a membranous and/or cytoplasmic marker with expression in normal epithelia and even in epithelial tumours. Despite wide distribution, it is useful as part of a panel in determining primary site of metastatic carcinoma in tissue and generally expressed with little variation in adenocarcinoma of lung, breast, thyroid, endometrium, cervix, ovary, salivary gland and upper GI tract, urothelial carcinoma, papillary renal cell carcinoma and Paget disease, but usually negative in colorectal carcinoma, Merkel cell carcinoma, hepatocellular carcinoma, prostatic adenocarcinoma, adrenocortical tumours and squamous cell carcinoma according Kruti and Brandon (2020). This study observed the expression of protein CK7 in the control and those that were treated with Vitamin E and Phoenix dactylifera extracts, which is evidence of continuous cell proliferation and differentiation, whereas the gossypol-treated group showed reduced evidence of tissue regeneration an indication of hepatic injury according to Adrian and Stefan (2010) and Goldstein et al. (2001).
The molecular and cellular mechanisms that involve process of lipid peroxidation initiate or propagate liver injury and fibrosis. At the molecular level, the production of reactive oxygen species such as superoxide anion radical (O2-.) is an essential event in the formation of hydroxyl radical (OH-.), which can then abstract a hydrogen atom from cellular membrane lipids, resulting in the autocatalytic production of lipid radicals and the peroxidative degradation of membrane lipid this is according to Brante et. al. (2010), this also flow with this study, we observed increased in MDA activities and lowering of SOD activities and the expression of receptor of GSH in gossypol treated animals. This is otherwise not achieved in the animals treated with the antioxidant-like substances, as noted in the Vitamin E and Phoenix dactylifera extracts treated animals. GSH has been noted as a natural compound in the body: made from the amino acids glutamic acid, cysteine, and glycine, it plays a detoxification role in the body and usually occurs inside cells of the liver, kidney, intestines, and lungs. GSH has been associated with lipid peroxidation, because of its ability to combine with free radicals that may initiate lipid peroxidation, as well as reduced hydrogen peroxide formed in cells according to Singh et al. (2013). Hepatic GSH has been observed to decrease according to gossypol concentration and the consequent depletion in the antioxidant enzymes as noted in this study. However, increase in the antioxidant enzymes was observed in the Vitamin E and Phoenix dactylifera extracts suggesting the antioxidant promoting capacities of the Phoenix dactylifera extracts in gossypol damage wistar rats.
The study of cytokeratin (CK) expression in liver tissues has offered valuable findings on hepatic ultrastructure in healthy and disease states. Normal functioning liver cells, hepatocytes express different forms of cytokeratin in healthy and diseased states. In healthy liver tissue, hepatocytes expressed CK8 and CK18 but in diseased condition, liver tissue have been reported to express CK7 and CK19 (Adrian and Stefan, 2010; Goldstein et al., 2001). CK7 immunohistochemistry conducted on liver tissue showed numerous CK7 hepatocytes especially on gossypol treated groups. This implies that gossypol may cause serious damage to liver tissue when abused and/taken over a long period of time.
Gossypol has been documented to cause oxidative stress in exposed subjects: i.e., irregularity in the production and clearance of free radicals that can be detrimental to hepatocytes health and functionality. Findings from this study showed a slight increment in an oxidative stress marker, MDA in the liver of gossypol treated animals and a likewise insignificant reduction of MDA levels in Phoenix dactylifera and vitamin-E-treated animals compared to gossypol group. Excess of oxidative stress markers in cells tend to affect the production and function of natural cellular oxidants such as SOD, GSH, etc. While the concentration of GSH seems unaffected across all experimental groups, SOD concentration was reduced significantly. This implies that the possible mechanism gossypol exerted its debilitation actions on the liver was via oxidative stress induction. Our finding is contrary to Wang et al. (2008) who reported that gossypol has anti-oxidative properties. Also, Mobasher et al. (2018) reported that Phoenix dactylifera L., via its antioxidative and antihyperlipidemic activities, reverted the azithromycin toxicity exerted on the hepatic and renal cyto-architecture and functions. Phoenix dactylifera and vitamin E were unable to significantly upregulate cellular oxidant (SOD & GSH) production in gossypol-treated animals.
Glucose-6-phosphate dehydrogenase (G6PDH) is an enzyme that maintains supply of reducing energy to body cells to aid cell growth and proliferation. It also protects RBC against oxidative damage (Tian et al., 1998; Thomas et al., 1991). Lactate dehydrogenase (LDH) is well expressed in body tissues. It is not released except in response to assault or tissue damage, and hence it is a common marker for injuries and diseases. Our findings revealed that gossypol altered G6PDH concentration in all gossypol-exposed groups, lowest in 30mg/kg gossypol group, which may impact hepatocellular health and functions.
Conclusion
Gossypol at dosages and duration tested in sexually active female rats showed debilitating effects on liver health and functions, and Phoenix dactylifera could not completely revert the toxic actions of gossypol on liver tissue.