This study aimed to examine the morphometry of the biceps brachii muscle in fetal cadavers, to reveal the difference between genders and sides, and to determine the variations. Our study was conducted on 47 upper extremities of 25 human fetuses (13 females, 12 males) who were aged between 29-40 weeks of gestation and had no external pathology and anomaly. The development of the determined parameters according to gestational age (month) was evaluated. Comparisons were made between genders and sides. In our study, no statistically significant difference was found in the footprint length of the biceps brachii muscle according to gestational age. Other parameters increased statistically significantly according to gestational age. No difference was found between genders in morphometric parameters. There was no statistically significant difference between the right and left extremities in other parameters, except for motor entry point. Motor entry point was found to be statistically significantly more distal in the left extremities compared to the right extremities (p=0.012). Furthermore, all parameters were positively correlated with gestational age. We considered that they would contribute to the correct diagnosis and treatment of pathologies related to this region especially in the intrauterine period, premature birth and in the newborn, as well as to the reduction of complications that may occur after treatment.
Investigation of the morphometry and variations of the biceps brachii muscle ın fetal cadavers
Semra Akgün, Kenan Öztürk, Ahmet Dursun, Yadigar Kastamoni
Department of Anatomy, Faculty of Medicine, Suleyman Demirel University, 32260, Isparta, Turkey
SUMMARY
Sign up or Login
INTRODUCTION
The biceps brachii muscle (BBM) is a thick, fusiform muscle that is located in the anterior part of the arm and passes both in front of the shoulder and elbow joints. The BBM consists of a long head originating from the supraglenoid tubercle proximally and a short head originating from the coracoid process. The long head of the BBM provides stabilization of the shoulder joint in dynamic movements such as throwing. Furthermore, the BBM reduces stress on the lower glenohumeral ligament (Szpinda et al., 2013).
While a large part of the distal tendon of the BBM adheres to the ulnar/posterior side of the radial tuberosity, a part of it is mixed with the lacertus fibrosus (LF). The LF is the thickening of the brachial fascia that covers the proximal part of the flexor-pronator muscle groups of the forearm and joins the BBM to the ulna. In distal tendon ruptures of the BBM, the LF can also prevent the retraction of the BBM toward the proximal if it is intact (Szpinda et al., 2013; Brigido et al., 2013).
The BBM is one of the most variable muscles in the body due to its variation and morphology. The variations related to the absence of the short or long head of the BBM and insertion are rare. The absence of the long head may reduce the flexion and supination strength of the forearm. Furthermore, since the tendon of the long head is used as a landmark in shoulder arthroscopy, the absence of this head may lead to difficulties during arthroscopy (Rodríguez-Niedenführ et al., 2003). Although the most frequently reported variation is the three-headed BBM, there are cases with up to seven heads reported in the literature (Rodríguez-Niedenführ et al., 2003; Nasr and Hussein, 2013). These variations are clinically important because accessory heads may mislead surgeons during shoulder operations or may lead to the compression of neurovascular structures (Rodríguez-Niedenführ et al., 2003). The third head of the BBM generally originates from the humerus between the attachments of the coracobrachialis and the brachialis muscles. Nevertheless, the third head can start from the coracoid process, the tendon or fascia of adjacent muscles, the intermuscular septum, shoulder joint capsule, head of humerus, neck of humerus, and anteromedial aspect of the humerus (Yildiz et al., 2006; Nayak et al., 2008; Angadi et al., 2016; Saluja et al., 2017).
Due to a large number and clinical significance of variations, studies on the BBM conducted in adult cadavers are quite common (Park et al., 2007; Athwal et al., 2007). However, information on the morphometry of the BBM in fetal cadavers is limited (Szpinda et al., 2013). We aimed to determine the morphometric development of the BBM in the fetal period, its variations and differences between genders and sides to assist surgical interventions on newborn and premature babies to be performed in this region in pathologies such as humerus fracture, neurovascular lesions, and tumors.
MATERIALS AND METHODS
Our study was conducted on 47 upper extremities of 25 human fetuses (13 females, 12 males) who were aged between 29-40 weeks of gestation and had no external pathology and anomaly, in the Faculty of Medicine, Anatomy Department Laboratory, which were obtained from the Maternity and Children’s Hospital between 1996-2014 by getting permissions from their families. Three extremities that were damaged during dissection and not suitable for the measurement were excluded from the study. Prior to the study, approval was obtained from the Faculty of Medicine Ethics Committee (Date: 03.05.2019, Decision No:83). The causes of death in fetal cadavers are unknown. All fetal cadavers were fixed by the arterial injection of 10% (v/v) formaldehyde solution into the water and stored in a pool of 10 L of 10% (v/v) formaldehyde solution. It is a known fact that formaldehyde has a shrinkage effect on tissues. In a study on human muscles, this effect was reported to be 2.22% on average (Cutts, 1988). The 2.22% effect should be considered in the evaluation of the data in the publication.
The gestational age of the fetuses was determined according to the Crown Rump Length (CRL), Bi-Parietal Diameter (BPD), Head Circumference (HC), and Femur Length (FU). The fetuses were evaluated by being divided into three groups. as the 8th month fetuses between the weeks 29-32, 9th month fetuses between the weeks 33-36, and 10th month fetuses (full term) between the weeks 37-40.
The anatomical dissection method was used in all fetal cadavers to determine the parameters of the BBM. First, an incision was made from the outer end of the clavicle, ending at the lower edge of the anterior wall of the axilla. Second, an incision was made from the midpoint of first incision and ending at the interepicondylar line. The skin and subcutaneous adipose tissue were removed. The deltoid and pectoralis major muscles were released as needed by entering through the deltopectoral range in order to see the origo of the long and short heads. The skin and subcutaneous adipose tissue of the proximal forearm were removed by making another incision from the interepicondylar line to the distal of the cubital fossa to determine the insertion of the BBM. The LF was identified and photographed with a millimeter ruler. Afterward, the LF was incised, and deep structures were uncovered. Under the “EUROMEX Edublue 1805-S binocular digital stereo microscope” (EUROMEX microscopen BV, Arnhem, Holland), the insertion of the BBM was evaluated at 10X magnification. The morphometric measurements were performed using a digital caliper with a precision of 0.01 mm. The area of the photographed LF was measured using the Image J analytical software (National Institutes of Health, Bethesda, MD) program.
For each extremity, the following parameters were examined:
Arm length: distance between the acromion and olecranon while the arm in extension.
Long head length: distance between the origin and common muscle belly. The joint capsule was opened and the supraglenoid tubercle was found. Long head length was measured from the beginning of the long head to the common muscle belly with an inflexible rope. Then the length of this rope was determined with a digital caliper.
Long head width: the widest level of the long head.
Short head length: distance between the origin and common muscle belly.
Short head width: the widest level of the short head.
Common muscle belly length: distance between the start of the common muscle belly and musculotendinous junction.
Common muscle belly width: the widest level of the common muscle belly.
Motor entry point (MEP): distance between the acromion and point where the motor nerve enters the muscle.
Footprint length: the length of the attachment of the distal tendon to the radial tuberosity.
Area of the lacertus fibrosus: firstly, lacertus fibrosus was identified. Then, its perimeter was drawn with the Image J program and its area was measured.
Number of distal tendons and the localization of these tendons according to the radial tuberosity.
Statistical analysis was performed using SPSS Inc. SPSS for Windows 20.0 statistical package program. Since the MEP data were not normally distributed, the Kruskal-Wallis test and Mann-Whitney U test, which are among the non-parametric tests, were used to analyze the data, and Spearman’s Correlation test was used in the correlation analysis. Since other data were normally distributed, the Independent Samples T-Test and One-Way ANOVA test, which are among the parametric tests, were used to analyze the data, and Pearson’s Correlation test was used in the correlation analysis. The significance level was considered to be p<0.05 in statistical analysis.
RESULTS
In our study, no statistically significant difference was found in the footprint length of the BBM according to gestational age (month). Other parameters increased according to gestational age, which was statistically significant. There was no statistically significant difference between the right and left extremities in other parameters, except for MEP. The MEPs were found to be statistically significantly more distal in the left extremities compared to the right extremities (p=0.012) (Table 1).
The averages and standard deviations of the ratios of parameters related to the BBM to arm length according to sides, gender, and gestational age are presented in Table 2. When Table 2 was analyzed, statistically significant differences (p=0.009 and p=0.012, respectively) were observed in the common muscle belly width/arm length and motor entry point/arm length ratios according to gestational age. While it was found that the difference between months in the common muscle belly width/arm length ratio resulted from the 8th-10th months, the difference in the motor entry point/arm length ratio resulted from the 8th-9th and 8th-10th months. The ratio of MEPs to the arm length measured between the acromion and olecranon was found to be 0.37±0.04 on average in all specimens, and these points were determined to be in the middle third of the arm. In the right-left sides comparison of the motor entry point/arm length ratio, it was observed to be statistically significantly higher in the left extremities compared to the right extremities (p=0.023). In the gender comparison of the long head width/arm length ratio, it was found to be higher in female fetal cadavers compared to male fetal cadavers (p=0.039).
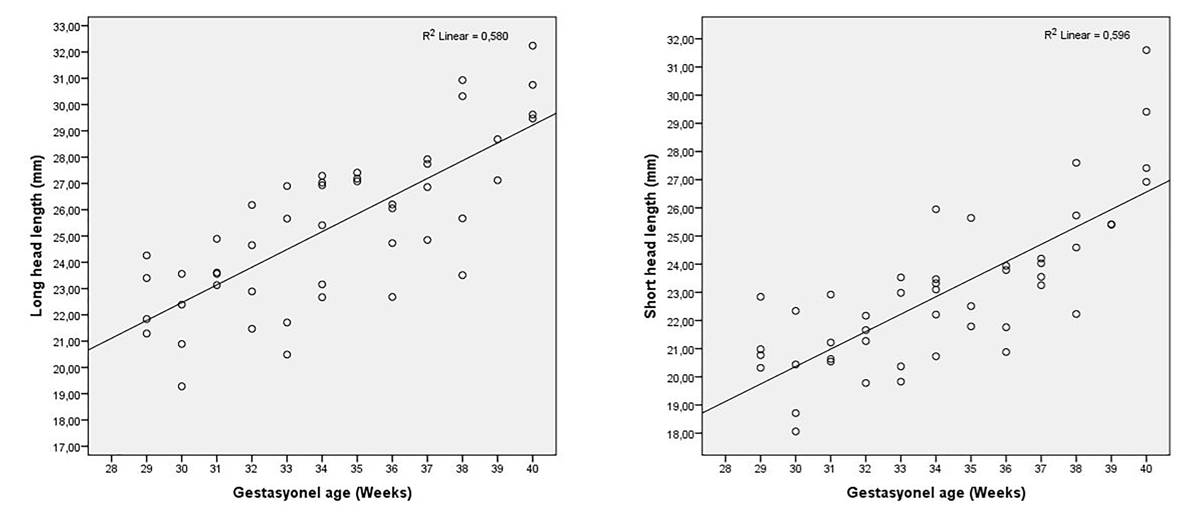
All parameters were positively correlated with gestational age (week) (Table 3). The parameters with the best positive correlation according to gestational age (week) were the short head (r=0.772) and long head (r=0.762) lengths, respectively (Fig. 1). When the correlation of the parameters with respect to each other was examined, the long head and short head lengths showed the strongest correlation (r=0.908) (Table 3).
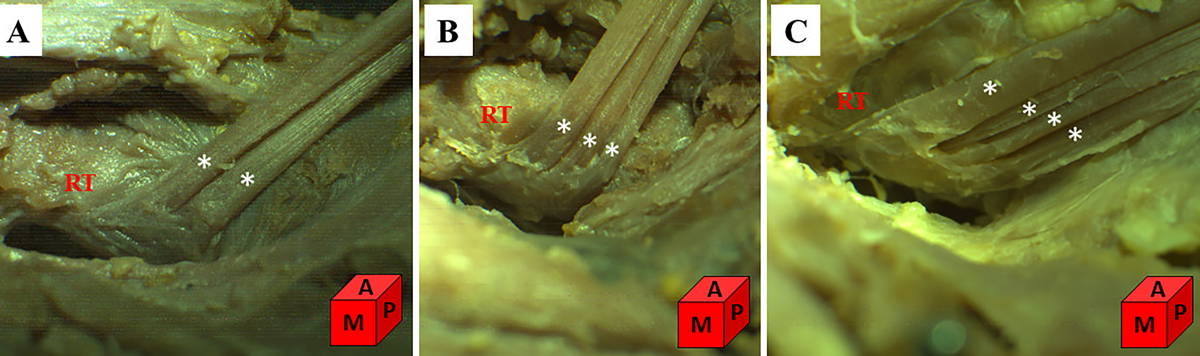
The distal tendon was evaluated in 46 of 47 upper extremities in our study. In a specimen, the distal tendon could not be evaluated because it was damaged in dissection. In all specimens, it was observed that the distal tendon adhered to the ulnar/posterior side of the radial tuberosity. While two tendons were observed in 60.8% (n=28) of the specimens, three tendons and four tendons were observed in 34.7% (n=16) and 4.34% (n=2) of the specimens, respectively (Fig. 2). In our study, we found the accessory head variation of the BBM in 2 (4.2%) of 47 upper extremities. It was observed that the accessory head, which was located bilaterally in the same fetus, was located medial to the short head and anterolateral to the coracobrachialis muscle, and originated from the coracoid process with the short head (Fig. 3).
DISCUSSION
Warmbrunn et al. (2018) reported that all muscles developed similarly to adult morphology by the 8th week of the embryonic period. The age of the fetuses used in our study starts from the 29th week of gestation. Therefore, the BBM of the fetuses in our study completed its morphological development.
The BBM is an important muscle of the anterior part of the arm. Studies on the BBM are usually related to lesions, the repair of lesions, and variations of the BBM (Nasr and Hussein, 2013; Walton et al., 2015). Studies examining the morphometry of the BBM from proximal to distal in fetal cadavers are limited (Szpinda et al., 2013).
In their study on the normal development of the BBM conducted in 17-30 weeks old fetal cadavers, Szpinda et al., (2013) reported that the dynamic development of the BBM was linear. They indicated that there was no significant difference between the right and left sides and genders in the parameters examined. They reported that there was a statistically significant correlation between each parameter studied and gestational age and that all parameters increased with gestational age. Likewise, in our study, it was found that the parameters were positively correlated with gestational age.
The variations observed in the origo of the BBM are quite common. The variations of its insertion are rarer (Nasr and Hussein, 2013). The third head of the BBM generally originates from the humerus between the attachments of the coracobrachialis and the brachialis muscles (Vázquez et al., 2003; Yildiz et al., 2006; Kervancioglu and Orhan, 2011; Angadi et al., 2016). Embryologically, the third head of the BBM is considered as a piece separated from the brachialis muscle and migrated to the insertion with the musculocutaneus nerve. The presence of an accessory head with or without neural variations may be caused by a change in the signal between a group of mesenchymal cells and the neuronal growth factors of the forearm muscles developing from the paraxial mesoderm or the presence of circulatory factors during the formation of the brachial plexus (Nasr and Hussein, 2013; Angadi et al., 2016).
In our study, an accessory head variation was found bilaterally in 1 (4%) of 25 fetal cadavers (Fig. 3). It was observed that the accessory head was located medial to the short head and anterolateral to the corocobrachialis muscle, and started from the coracoid process with the short head. The incidence of the accessory head was reported between 3.3-37.5%, although it varied by races (Schwerdtfeger et al., 2018). The prevalence of the accessory head was shown to be 8% in Chinese, 10% in white Europeans, 12% in black Africans, 15% in Turkish, 18% in Japanese, and 37.5% in Colombians (Yıldız et al., 2006; Nasr and Hussein, 2013; Angadi et al., 2016; Saluja et al., 2017; Schwerdtfeger et al., 2018). The incidence of bilateral symmetrical accessory heads was reported less often (Nakatani et al., 1998; Saluja et al., 2017). In the majority of cases, accessory heads are asymptomatic and detected randomly during surgery or imaging. However, in some cases, these accessory heads may lead to clinical symptoms (Kervancioglu and Orhan, 2011). For instance, the accessory heads of the BBM may lead to high median nerve entrapment symptoms, thrombosis, and edema by compressing neurovascular structures. Functionally, they change the flexion and supination strength of the forearm. Furthermore, unilateral variations can be mistakenly interpreted as soft tissue tumors during magnetic resonance imaging (Kervancioglu and Orhan, 2011; Saluja et al., 2017).
The BBM and its accessory head are known to be usually innervated by the musculocutaneus nerve (Park et al., 2007; Schwerdtfeger et al., 2018). In our study, it was determined that all BBM and accessory heads were also innervated by the musculocutaneus nerve. Park et al. (2007) determined a reference line between the lateral epicondyle of the humerus and the coracoid process in order to identify the motor point of the musculocutaneus nerve innervating the BBM in adult cadavers. They identified that the MEP of the BBM was about half of this reference line. In their study conducted with fetuses with gestational ages ranging from 16 to 36 weeks, Kervancioğlu et al. (2011) measured the distance between the motor branches of musculocutaneus nerve and the acromion. For the BBM, they indicated that the average distance between the MEP of all motor branches and the acromion was 3.8±6.1% of the acromion-lateral epicondyle length. In our study, the average length of the BBM’s MEPs to the acromion was found to be 27.73±4.22 mm. The MEPs were found to be proximal 37±4% of the arm length.
The LF is the thickening of the brachial fascia that connects the BBM to the ulna and covers the proximal part of the flexor-pronator muscle group. The LF is very important clinically. The LF located in the fossa cubitalis protects the neurovascular structures under it. Depending on the muscle activity in the forearm, it provides proprioceptive information for the BBM. It also has additional anatomical support for the BBM tendon (Caetano et al., 2017). In repairing the LF rupture, suturing the LF should be performed in pronation and extension, which could also minimize the risk of impingement of the underlying neurovascular structures (Eames et al., 2007). Besides, the LF can be used as a local graft source for chronic distal biceps tendon ruptures (Hamer and Caputo, 2008). So, it is very important to know the area of LF. In the literature review we performed to make a comparison, while it was observed that there were studies on the LF conducted in adult cadavers (Athwal et al., 2007; Caetano et al., 2017), we did not find any study on the LF in fetuses. Therefore, in our study, the area of the LF was examined and found to be 35.45±8.62 mm2 on average. No statistically significant difference was found in the area of the LF by gender and sides.
The BBM main tendon takes an oval shape as it goes distally and turns from the frontal plane to the sagittal plane on itself and adheres to the radial tuberosity. Although it was classically defined in this way, in recent studies, it has been observed that the distal biceps tendon consists of two separate tendons, one belonging to the short head and one belonging to the long head, in most of the people (Eames et al., 2007). Nowadays, avulsion rupture of the distal biceps tendon is reported more frequently than before, and surgical methods are used for the repair of these ruptures (Safran and Graham, 2002; Hutchinson et al., 2008; Walton et al., 2015). If the biceps tendon is not fully positioned in its anatomical position and is positioned only in the center of the radial tuberosity during surgery, supination strength can never reach its pre-injury strength (Hutchinson et al., 2008). Knowledge of the number and location of the distal tendons can assist surgeons during surgical repair of distal biceps tendon ruptures. Nevertheless, in the literature, there are few definitions of the morphometry or variations of the distal biceps tendon. However, information about the distal biceps tendon is important in deciding the most appropriate method to be applied during the surgical procedure, such as in which case a graft supplement should be used to complement the natural tendon and what the most suitable graft should be (Walton et al., 2015). Therefore, we consider that our study will provide significant data in this regard.
In their study on 15 upper extremities of adult cadavers, Athwal et al. (2007) demonstrated that the long head and short head continued completely separately without joining in the common muscle belly in two of the specimens and that distal tendons ended separately. They added that the long head ended proximally in the radial tuberosity and the short head ended distally. In eight of the specimens, they indicated that the muscle belly, the distal tendons corresponding to the long head and short head could be easily separated, and that the long head ended proximally in the radial tuberosity and the short head ended distally. In five of the specimens, they demonstrated that the long head and short head formed a common muscle belly by combining distally, and that the corresponding distal tendons were also combined. In these specimens, it was indicated that the muscle bellies could be separated by minimal dissection; however, the detection of the distal tendon footprint was difficult. Furthermore, the researchers reported that the distal biceps tendon was attached along the ulnar/posterior side of the radial tuberosity in all specimens. Likewise, in our study, the distal tendon of the BBM was attached to the ulnar/posterior side of the radial tuberosity in all specimens. While two tendons were observed in 60.8% (n=28) of the extremities, three tendons and four tendons were observed in 34.7% (n=16) and 4.34% (n=2) of the extremities, respectively. In our study, although the distal tendons of the BBM could be observed separately from each other, it was not possible to determine to which head these tendons originating from the common muscle belly belonged.
The BBM insertion is on the ulnar/posterior side of the radial tuberosity and creates a footprint on the bone (Athwal et al., 2007; Hutchinson et al., 2008). The localization of the footprint is important for the strength of supination. Several authors have reported a significant loss of supination range and strength after the distal biceps tendon repair with an anteriorly located footprint on the radial tuberosity, and have noted that a posteriorly located footprint repair is important for the restoration of supination torque (Van den Bekerom et al., 2016). Therefore, having knowledge about the dimensions and localization of footprints is important for minimizing complications during and after the surgical repair of the distal biceps tendon ruptures. The reported sizes of the distal biceps tendon insertional footprint on the radial tuberosity vary and exhibit gender differences. However, it was reported that there was no difference between the dimensions of the right and left footprints (Athwal et al., 2007; Van den Bekerom et al., 2016). In our study, the footprint length was found to be 2.85±0.6 mm on average. No statistically significant difference was determined in the footprint length between the right and left sides and genders.
The pathology of the distal biceps brachii tendon is less common than the pathology of the proximal biceps brachii tendon in the shoulder. Nowadays, the incidence of the distal biceps brachii tendon rupture is increasing due to the widespread use of ultrasound and magnetic resonance imaging for diagnosis (Brigido et al., 2013). Therefore, along with the developing technology, it has become more important to know the anatomy of the distal biceps brachii tendon (distal tendon, radial tuberosity, footprint and lacertus fibrosus) in order to understand the pathomechanism, optimize the treatment outcome, and prevent complications while repairing the distal biceps tendon.
Having a good knowledge of the anatomy of the BBM provides a better understanding of the lesions involving the shoulder and elbow and facilitates the planning of their treatment. The data obtained in our study are considered to contribute to the studies in the fields of anatomy, obstetrics, orthopedy, surgery, radiology, and pediatrics in the determination of anomalies, pathologies, and variations related to the BBM in the fetal period.
AKNOWLEDGEMENTS
We thank Prof. Soner Albay for his contributions.
Related articles
ANGADI MB, TANDON A, PANDIT S, BHATNAGAR R (2016) Supernumerary head of biceps brachii and branching pattern of the musculocutaneous nerve. Med J DY Patil Univ, 9: 264-266.
ATHWAL GS, STEINMANN SP, RISPOLI DM (2007) The distal biceps tendon: footprint and relevant clinical anatomy. J Hand Surg Am, 32: 1225-1229.
BRIGIDO MK, DE MAESENEER M, MORAG Y (2013) Distal biceps brachii. Semin Musculoskelet Radiol, 17: 20-27.
CAETANO EB, VIEIRA LA, ALMEIDA TA, GONZALES LAM, BONA JE, SIMONATTO TM (2017) Bicipital aponeurosis. Anatomical study and clinical implications. Rev Bras Ortop, 53: 75-81.
CUTTS A (1988) Shrinkage of muscle fibres during the fixation of cadaveric tissue. J Anat, 160: 75-78.
EAMES MH, BAIN GI, FOGG QA, VAN RIET RP (2007) Distal biceps tendon anatomy: a cadaveric study. J Bone Joint Surg Am, 89: 1044-1049.
HAMER MJ, CAPUTO AE (2008) Operative treatment of chronic distal biceps tendon ruptures. Sports Med Arthrosc Rev, 16: 143-147.
HUTCHINSON HL, GLOYSTEIN D, GILLESPIE M (2008) Distal biceps tendon insertion: an anatomic study. J Shoulder Elbow Surg, 17: 342-346.
KERVANCIOGLU P, ORHAN M (2011) An anatomical study on the three-headed biceps brachii in human foetuses, and clinical relevance. Folia Morphol (Warsz), 70: 116-120.
KERVANCIOGLU P, ORHAN M, KILINC N (2011) Patterns of motor branching of the musculocutaneous nerve in human fetuses and clinical significance. Clin Anat, 24: 168- 178.
NAKATANI T, TANAKA S, MIZUKAMI S (1998) Bilateral four-headed biceps brachii muscles: the median nerve and brachial artery passing through a tunnel formed by a muscle slip from the accessory head. Clin Anat, 11: 209-212.
NASR AY, HUSSEIN AM (2013) Morphology and clinical implication of the extra- head of biceps brachii muscle. Folia Morphol (Warsz), 72: 349-356.
NAYAK SR, KRISHNAMURTHY A, KUMAR M, PRABHU LV, SARALAYA V, THOMAS MM (2008) Four-headed biceps and triceps brachii muscles, with neurovascular variation. Anat Sci Int, 83: 107-111.
PARK BK, SHIN YB, KO HY, PARK JH, BAEK SY (2007) Anatomic motor point localization of the biceps brachii and brachialis muscles. J Korean Med Sci, 22: 459-462.
RODRÍGUEZ-NIEDENFUHR M, VÁZQUEZ T, CHOI D, PARKIN I, SAÑUDO JR (2003) Supernumerary humeral heads of the biceps brachii muscle revisited. Clin Anat, 16: 197-203.
SAFRAN MR, GRAHAM SM (2002) Distal biceps tendon ruptures: incidence, demographics, and the effect of smoking. Clin Orthop Relat Res, 404: 275-283.
SALUJA S, DAS SS, KUMAR D, GOSWAMI P (2017) Bilateral three-headed biceps brachii muscle and its clinical implications. Int J Appl Basic Med Res, 7: 266-268.
SCHWERDTFEGER LA, PASCOE MA, CLAPP T (2018) High incidence of a third head of biceps brachii in females. Transl Res Anat, 12: 25-27.
SZPİNDA M, PARUSZEWSKA-ACHTEL M, DĄBROWSKA M, BADURA M, ELMINOWSKA-WENDA G, SOBOLEWSKA A, SZPINDA A (2013) The normal growth of the biceps brachii muscle in human fetuses. Adv Clin Exp Med, 22: 17-26.
VAN DEN BEKEROM MP, KODDE IF, ASTER A, BLEYS RL, EYGENDAAL D (2016) Clinical relevance of distal biceps insertional and footprint anatomy. Knee Surg Sports Traumatol Arthrosc, 24: 2300-2307.
VÁZQUEZ T, RODRÍGUEZ-NIEDENFUHR M, PARKIN I, SAÑUDO JR (2003) A rare case of a four-headed biceps brachii muscle with a double piercing by the musculocutaneous nerve. Surg Radiol Anat, 25: 462-464.
WALTON C, LI Z, PENNINGS A, AGUR A, ELMARAGHY A (2015) A 3-dimensional anatomic study of the distal biceps tendon: implications for surgical repair and reconstruction. Orthop J Sports Med, 3(6): 2325967115585113.
WARMBRUNN MV, DE BAKKER BS, HAGOORT J, ALEFS‐DE BAKKER PB, OOSTRA RJ (2018) Hitherto unknown detailed muscle anatomy in an 8-week-old embryo. J Anat, 233: 243-254.
YILDIZ E, ALBAY S, YAZAR F, KIRICI Y, OZAN H (2006) M. Biceps brachii’nin aksesuar humeral başı: Olgu sunumu. S.D.Ü. Tıp Fak Derg, 13: 31-33.