Diabetes mellitus is a chronic metabolic disorder that adversely affects male reproductive organs, resulting in infertility due to testicular atrophy. Hyperglycemia and oxidative stress are considered the main factors implicated in the pathogenesis of diabetic complication. Hesperidin is a natural flavonoid with wide pharmacological effects such as hypoglycemic, antioxidant and anti-inflammatory. This work aims to study the ability of hesperidin to counteract diabetes-induced testicular alterations. 50 rats were used in this experiment, divided equally and randomly into five groups: control C, diabetic DM, diabetic +hesperidin DM+H, diabetic +insulin DM+In and hesperidin group H. At the end of the experiment, the rats were sacrificed and evaluated for body weight, blood sugar level, testicular weight, histopathological, immunohistochemistry, morphometric analyses, and spermatozoa analysis. Results revealed that DM significantly reduced the body and testicular weights, produced drastic histopathological, morphometric adverse changes of the testicular tissues and morphological abnormalities of the spermatozoa. Hesperidin produced valuable hypoglycemic effect, increased the body and testicular weights, ameliorated the histopathological changes, restored normal germinal epithelium and spermatogenesis, restored morphometric parameters and significantly decreased the morphological abnormalities of the spermatozoa caused by DM. Insulin could improve some parameters that were adversely changed by DM, but less than the hesperidin. We conclude that treatment with hesperidin appeared to be more effective in counteracting the toxic effects of diabetes on testes than insulin.
Influence of each hesperidin and insulin on diabetes-induced testicular alterations in adult albino rats
Maha S. Abd Elsamie, Mona H. Mohammed Ali, Omayma M. Mahmoud, Eman M. Kamel
Anatomy Department, Faculty of Medicine, Suez Canal University, Egypt
SUMMARY
Sign up or Login
INTRODUCTION
Diabetes mellitus is associated with reproductive impairment in both men and women. Its impact on reproduction can be profound, by a decrease in fertility and increase in reproductive failures (Ramalho-Santos et al., 2008). Male reproductive functions can be affected at multiple levels, including increased apoptosis in testicular germ cells, altered spermatogenesis, variation in sperm quality and quantity, decreased testicular weight, and ejaculatory dysfunction and reduced libido (Ricci et al., 2009; Jain and Jangir, 2014). Men with DM have low testosterone levels, associated with decreased luteinizing hormone and follicle-stimulating hormone concentrations. Therefore, diabetes-induced reproductive dysfunction is an important challenge (Ebong et al., 2014). Many articles explained that diabetic complications are due to hyperglycemia and overproduction of reactive oxygen species (ROS) that exceeds natural antioxidant defenses of the body, resulting in cell apoptosis (Ghlissi et al., 2012). Both testicular and sperm cells have increased susceptibility to free radical damage due to higher levels of polyunsaturated fatty acids, low oxygen tension and lack of antioxidant defense mechanisms (Idris et al., 2012). Many natural products have hypoglycemic activity and significant antioxidant capacity (Ebong et al., 2014).
Intake of citrus fruits and their constituting flavonoids can reduce the risk of certain chronic diseases and increase survival (Jasmin and Jaitak, 2019). Hesperidin is the predominant flavonoid in lemons and oranges. Hesperidin had a wide range of biological effects including hypolipidemic, antioxidant, anti-inflammatory, hypoglycemic, anticarcinogenic activities and has anti-apoptotic efficacy (Elshazly et al., 2018). Hesperidin showed significant antioxidant potential in terms of scavenging free radicals produced by various in vitro assays. Hesperidin showed optimum protection against free-radical-induced cellular damage (Kalpana et al., 2009). Moreover, studies have shown that hesperidin is nontoxic and causes no allergic reactions in male or female mice (Acipayam et al., 2014). So, the present study was conducted in order to compare the effects of hesperidin and insulin in counteracting diabetes-induced testicular alterations in adult albino rats.
MATERIAL AND METHODS
Animals
Fifty adult male Sprague Dawley albino rats weighing 200-250 g were obtained from the animal house of Faculty Veterinary Medicine, Suez Canal University. The rats were acclimatized two weeks before drug administration, housed in a separate cage (10 rats each). The experiment was performed at the Animal and Experimental House, Anatomy department, Faculty of Medicine, Suez Canal University. All experiments were carried out in accordance with the guidelines of the Institutional Animals Ethics Committee of Suez Canal University.
Chemicals
Streptozotocin (STZ) and Insulin were purchased from Sigma pharmaceutical, Egypt. Hesperidin was purchased from Sigma Chemical, Co., St. Louis, Mo., USA
Experimental design
The rats were randomly divided into five groups (10 rats each), as follows:
• Group I: Control group (C), which was subdivided into:
Group IA: rats received nothing and served as negative control.
Group IB: rats received distilled water (the vehicle for hesperidin) orally, through an oral gavage, at a dose of 200 mg/kg/day for 10 consecutive days and served as positive control I (Arafa et al., 2009).
Group IC: rats received citrate buffer (the vehicle for STZ) intraperitoneally at a dose of 40 mg/kg just once and served as positive control II (Kassab et al., 2019; Ricci et al., 2009).
• Group II: Diabetes-induced group (DM), rats received STZ dissolved in citrate buffer intraperitoneally at a dose of 40 mg/kg just once. Seven days after STZ injection, rats were screened for fasting blood glucose levels (through blood obtained from a puncture of the tail vein). The rats having fasting blood glucose levels higher than 200 mg/dl were selected for experimentation (Kassab et al., 2019).
• Group III: Diabetes-induced group treated with hesperidin (DM+H). Diabetic rats received hesperidin dissolved in distilled water orally, through an oral gavage, at a dose of 200 mg/kg/day for 10 consecutive days once hyperglycemia was confirmed (Arafa et al., 2009).
• Group IV: Diabetes-induced group treated with insulin (DM+In). Diabetic rats received insulin subcutaneously at a dose of 15 IU/kg daily for 10 consecutive days, once hyperglycemia was confirmed (Moir and Zammit, 1994).
• Group V: Hesperidin-treated group (H). Rats received hesperidin dissolved in distilled water orally, through an oral gavage, at a dose of 200 mg/kg/day for 10 consecutive days (Arafa et al., 2009).
Animals weighed daily, and drug doses were adjusted accordingly. Rats were sacrificed 24 hours after the last dose of drugs. Testes were extracted, weighed, fixed in Bouin’s solution, embedded in paraffin and sectioned at 5 µm thickness (Mahmoud et al., 2014). Left testes were used to obtain testicular weight, processed for histopathological staining, immunohistochemical and morphometric measurements.
Immunohistochemistry
- Proliferating cell nuclear antigen (PCNA): Specific monoclonal anti- PCNA antibody was used to estimate the degree of cell proliferation. Positive PCNA labeling was detected in cells located at or near the basement membrane of the seminiferous tubules (Kanter et al., 2012).
- B cell leukemia/lymphoma-2 (Bcl-2): Bcl-2 is an anti-apoptotic protein. It expressed in most tubular cells with preferential expression in cytoplasm of the spermatids close to the luminal surface (Oldereid et al., 2001).
Morphometry
The seminiferous tubules’ slides were examined at x400 magnification, captured using a light microscope coupled to a digital camera (Olympus, Japan). Thirty seminiferous tubules were randomly chosen and analyzed with ImageJ software (Schneider et al., 2012; Mahmoud et al., 2014; Kianifard et al., 2011). H&E-stained sections were used for measuring thickness of tunica albuginea, diameter of the seminiferous tubules, thickness of the basement membrane of the seminiferous tubules and seminiferous epithelium height (μm). PCNA-stained sections were analyzed for measuring the number of proliferating nuclei (Kanter et al., 2012). Bcl-2-stained sections of the testis were analyzed for measuring percentage area positively stained with Bcl-2 protein (Schiller et al., 2002).
Preparation of rat sperms
Spermatozoa were collected from rats’ left cauda epididymis, according to methods described by (Suresh et al., 2010).
Statistical analysis
Results were analyzed using the Statistical Package of Social Science (SPSS) computer software, version 21. All data were reported as mean ± standard deviation (SD). To evaluate significant differences, the comparison of means between each two experimental groups was done by One-way analysis of variance (ANOVA) and Post hoc Bonferroni tests. P value <0.05 was considered statistically significant and P value <0.01 was considered highly statistically significant.
RESULTS
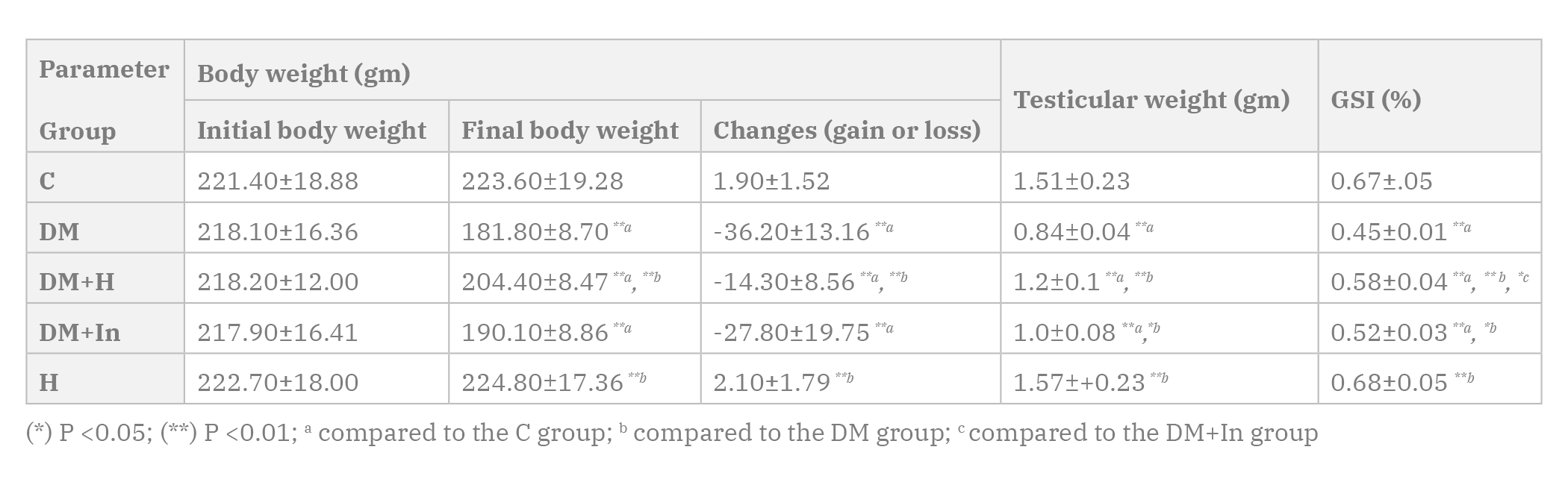
Body weight, testicular weights and GSI
The rats in the DM, DM+H and DM+In groups showed a highly statistically significant decrease in the final body weight, testicular weight and GSI when compared to the C group. The final body weight and testicular weight of the rats in the H group show statistically insignificant difference when compared to the C group (Table 1).
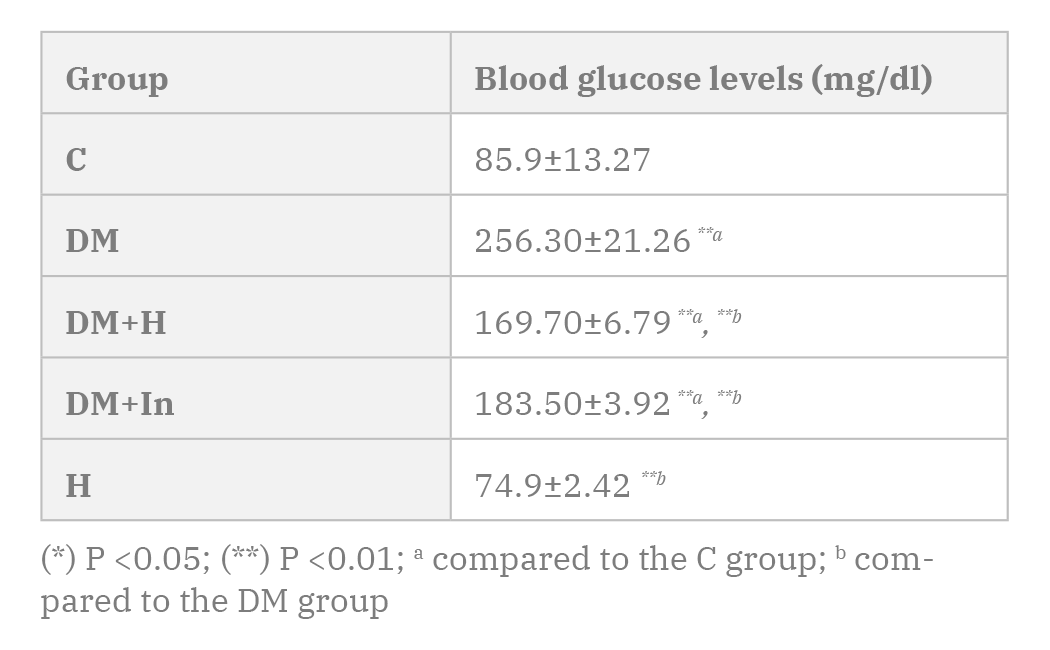
Blood glucose levels
The rats in the DM, DM+H and DM+In groups showed a highly statistically significant increase in the blood glucose levels when compared to the C group. DM+H and the DM+In groups showed a highly statistically significant decrease in the blood glucose levels when compared to the DM group (Table 2).
Histopathological results of the testicular tissues
Control group:
The rat testes of the C group showed a normal histological architecture. Homogenous tunica albuginea surrounded closely packed seminiferous tubules, enveloped with normal basement membrane and myoid cells having flat nuclei. The tubules were lined by multiple layers of distinct germinal epithelial cells with normal shape and size. Spermatogonia appeared small with oval nucleus. 1ry spermatocytes appeared as the largest cells of all spermatogenic cell series, having large vesicular nucleus. 2ry spermatocytes were of a small size and vesicular nucleus. Spermatids also appeared as small cells with vesicular nucleus. Normal spermatozoa appeared filling the lumen of the seminiferous tubules. Sertoli cells, extending between the basal lamina and the tubular lumen, were normal in size and having oval nuclei. The interstitial tissue showed clusters of Leydig cells with distinct round nucleus and pale cytoplasm. Interstitial blood vessels were of normal wall thickness and normal diameter (Fig. 1).
PCNA: PCNA-positive cells were markedly detected in basal tubular compartment, presenting a single layer of brown PCNA-positive nuclei along the basement membrane of the seminiferous tubules (Fig. 5).
Bcl-2: marked cytoplasmic expression of brown Bcl-2 protein in most tubular cells with preferential staining close to the tubular lumen (staining mainly spermatids) (Fig.6).
Hesperidin-treated group:
Sections of the testicular tissues of the H group showed no difference from the C group Diabetes-induced group:
The testes of the rats of the DM group showed a disrupted histological architecture. There was marked thickening of the tunica albuginea with cystic degeneration. The basement membrane surrounding the seminiferous tubules was also thickened. The seminiferous tubules decreased markedly in size. The germinal epithelium was strongly reduced in height with disrupted morphology, even atrophied in some tubules. Vacuolization was seen within the germinal epithelium. Marked decrease in the number of spermatozoa in the tubular lumen was detected. Some seminiferous tubules were even devoid completely of spermatozoa. The interstitial tissue showed marked edema and hemorrhage with dilated and congested thick-walled blood vessels. Leydig cells were remarkably damaged and decreased (Fig. 2).
PCNA: The number of brown PCNA-positive cells was more markedly decreased in the DM group than in the C group, even absent in some seminiferous tubules (Fig. 5).
Bcl-2: Testicular tissues obtained from the diabetic rats showed a weak expression of brown Bcl-2 protein in comparison to the C group (Fig. 6).
Diabetes-induced group treated with hesperidin:
Administration of hesperidin to the diabetic rats resulted in restoration of a near-normal morphology of the testicular tissues. The tunica albuginea was of homogenous morphology and appeared thinner than that observed in the DM group. The basal lamina surrounding the seminiferous tubules also appeared thinner when compared to that of the DM group. The germinal epithelium showed marked increased height when compared to the DM group. Almost all cell types of the germinal epithelium were noticed lining the seminiferous tubules. Spermatozoa were filling the tubular lumen. However, some vacuolization was still seen within the germinal epithelium. The interstitial tissue showed a decrease in the edema and hemorrhage, and the blood vessels were much less congested with thinner walls than those observed in the DM group. Leydig cells were increased (Fig. 3).
PCNA: There was a moderate increase in the expression of brown PCNA-positive cells in the testicular tissues of the DM+H group in comparison to the DM group (Fig. 5).
Bcl-2: There was a moderate increase in brown Bcl-2 protein expression in the testicular cells of the DM+H group in comparison to the DM group (Fig. 6).
Diabetes-induced group treated with insulin:
Testicular tissues of diabetic rats treated with insulin showed some improvement when compared to that of DM group. The tunica albuginea appeared thinner, although sub-capsular hemorrhage was observed. Many seminiferous tubules showed increased diameter and increased germinal epithelial height with restoration of the different types of cells; other tubules were lined with disorganized epithelium, devoid of spermatozoa and showed degenerative changes. Vacuolization was still seen within the germinal epithelium. Some interstitial edema and hemorrhage were still observed. The interstitial blood vessels showed congestion and thick walls. Increased number of Leydig cells was observed (Fig. 4).
PCNA: There was a mild expression of brown PCNA-positive cells in the testicular tissues of the DM+In group when compared to the DM group (Fig. 5).
Bcl-2: There was a mild expression of brown Bcl-2 protein in the testicular tissues of the DM+In group when compared to the DM group (Fig. 6).
Morphometric results
1. Thickness of the tunica albuginea:
The rats of the DM, DM+H and DM+In groups showed a highly statistically significant increase in the thickness of the tunica albuginea when compared to the C group. On the other hand, the DM+H and DM+In groups showed a highly statistically significant decrease when compared to the DM group. The DM+H showed a highly statistically significant decrease in the thickness of the tunica albuginea when compared to the DM+In group. In the H group, the thickness of the tunica albuginea shows statistically insignificant difference from the C group (Table 3).
2. Tubular diameter:
The rats in the DM, DM+H and DM+In groups showed a highly statistically significant decrease in the tubular diameter in comparison to the C group. The DM+H and DM+In groups showed a highly statistically significant increase in the tubular diameter when compared to the DM group. Moreover, the DM+H showed a highly statistically significant increase in the tubular diameter when compared to the DM+In group. The rats in the H group showed a statistically insignificant difference in the tubular diameter when compared to the C group (Table 3).
3. Germinal epithelial height:
The rats in the DM, DM+H and DM+In groups showed a highly statistically significant decrease in the germinal epithelial height when compared to the C group. The DM+H group showed a highly statistically significant increase in the germinal epithelial height when compared to the DM group, while the DM+In group showed a statistically significant increase when compared to the DM group. The DM+H showed a highly statistically significant increase in the germinal epithelial height when compared to the DM+In group. The germinal epithelial height of the H group shows statistically insignificant difference from the C group (Table 3).
4. Thickness of the tubular basement membrane:
The rats in the DM, DM+H and DM+In groups showed a highly statistically significant increase in the thickness of the tubular basement membrane when compared to the C group. The DM+H and DM+In groups showed a statistically significant decrease in the thickness of the tubular basement membrane when compared to the DM group. The DM+H showed a statistically significant decrease in the thickness of the tubular basement membrane when compared to the DM+In group. The thickness of the tubular basement membrane of the H group show statistically insignificant change from the C group (Table 3).
5. Number of PCNA-positive cells:
The rats in the DM, DM+H and DM+In groups showed a highly statistically significant decrease in the number of PCNA-positive cells in comparison to the C group. The DM+H and DM+In groups showed a highly statistically significant increase in the number of PCNA-positive cells when compared to the DM group. The DM+H showed a highly statistically significant increase in the number of PCNA-positive cells when compared to the DM+In group. The number of PCNA-positive cells of the H group did not show a statistically significant difference from the C group (Table 3).
6. Percentage area positively stained with Bcl-2:
The rats in the DM, DM+H and DM+In groups showed a highly statistically significant decrease in the percentage area positively stained with Bcl-2 in comparison to the C group. The DM+H and DM+In groups showed a highly statistically significant increase in the percentage area when compared to the DM group. The DM+H showed a highly statistically significant increase in the percentage area when compared to the DM+In group. The percentage area positively stained with Bcl-2 of the H group show a statistically insignificant difference from the C group (Table 3).
Sperm morphology
Normal sperm morphology was demonstrated in Fig. 7a. The normal sperm consists of head, neck, and tail. The current results showed a highly statistically significant decrease in the total number of normal spermatozoa in the DM, DM+H and DM+In groups when compared to the C group. Head abnormalities were in the form of an absent head (Fig. 7b), a flat hook-less head (Fig. 7c) and an amorphous head (Fig. 7d). Neck abnormalities were mostly represented as a bent neck, either a backward bent (Fig. 7e) or a forward bent (Fig. 7f). Tail abnormalities detected were either a bent tail (Fig. 7g) or a ring tail (Fig. 7h). Also, a single sperm may exhibit multiple abnormalities (Fig. 7i) (Table 4).
DISCUSSION
DM is one of the most common endocrine metabolic disease. It affects numerous organs, system complications and dysfunction, including reproductive system. Nearly 90% of patients with DM suffer from sexual dysfunction which includes impotence, reduced libido, impaired ejaculation, and impaired semen parameters. Hyperglycemia and oxidative stress were the main factors implicated in the pathogenesis of the diabetic complications. Oxidative stress in diabetic rats causes testicular DNA damage, depletion of spermatogenic cells and delay of spermatogenesis (Long et al., 2018). The current widespread hypoglycemic agents, besides causing several side effects, have no antioxidant effect and cannot prevent the progression of the diabetic complications (Ebong et al., 2014). Thus, a regimen that can cause hypoglycemia, target oxidative stress and improve diabetic complications is required.
In the current study, we compared the effect of hesperidin, a natural antioxidant, with the effect of insulin in counteracting testicular complications caused by DM.
The current results showed that the final body weight of the diabetic rats was reduced in a statistically significant way when compared to control group. Hesperidin administration increase body weight significantly while insulin did not significantly increase body weight of the rats. Weight loss of the rats observed in STZ-induced DM is not caused directly by the toxicity of STZ, but by the induction of DM (Jain and Jangir, 2014; Dkhil et al., 2016). Weight loss can be explained by loss of tissue proteins and increased muscle wasting that accompany DM (Cheng et al., 2013). STZ possibly prevents secretion of testosterone and growth hormone that results in disrupted anabolic activities, resulting in loss of weight (Long et al., 2018). The improvement of the body weight was attributed to the antioxidant effects of hesperidin and its capacity to enhance insulin secretion by pancreas, improve glucose metabolism, increase uptake of glucose by tissues and reverse most of the toxic effects of DM (Visnagri et al., 2014). Administration of exogenous insulin does not increase the rate of muscle protein synthesis (Sudha et al., 2000; Trommelen et al., 2015).
The current study showed a statistically significant decrease in the testicular weight of DM group. Administration of hesperidin caused a highly significant increase in the testicular weight. Administration of insulin increased the testicular weight significantly. Oxidative stress and hyperglycemia had a marked destructive effect on the testicular cells, causing apoptotic death, damage of the tubular epithelial lining led to marked thinning of the epithelium and atrophy. This destruction of the testicular interstitial tissue results in testicular atrophy, reduction of weight and dysfunction (Idris et al., 2012; Long et al., 2018). Administration of hesperidin increased the testicular weight significantly and ameliorated testicular weight loss (Arafa et al., 2009; Kaya et al., 2015). The protective effects of hesperidin could be ascribed to its antioxidant potentials through scavenging free radicals, improving the antioxidant defense mechanisms in testicular tissues, which in turn inhibit apoptosis, enhance spermatogenesis, regeneration of Leydig cells and restore testicular weight (Vijaya Bharathi et al., 2015). The restoration of testicular weight with insulin treatment is through its beneficial effect on the hypothalamic-pituitary-testicular axis, thus normalizing the secretion of testosterone by Leydig cells (Schoeller et al., 2012; Dkhil et al., 2016).
GSI is used to assess the damage to the testes in relation to the body (Latif et al., 2008). In the current study, the diabetic rats showed decrease in GSI when compared to the C group. Hesperidin treatment significantly increase GSI. Insulin treatment also showed a significant increase in GSI values, but less than hesperidin group. This may be due to the fact that testes were markedly affected in diabetic rats, and that the testicular weight decreased more than the total body weight (Sangameswaran and Jayakar, 2008). Controversially, some researchers found that GSI did not change in diabetic groups. This may be due to proportional decrease in the body and testicular weights. Hesperidin increase both body weight and testicular weight, thus increasing GSI (Alluanan Adelson do Nascimento et al., 2014; Ugarte et al., 2012).
STZ in this study produced a significant elevation of the blood glucose levels. Both hesperidin and insulin significantly lowered blood glucose levels. STZ hyperglycemia is explained by beta cell destruction and alters glucose metabolism (Ahmed et al., 2012; Ugarte et al., 2012). Hesperidin effects may be through attenuation of oxidative stress, decreasing production of pro-inflammatory cytokines, increasing the sensitivity of insulin receptors, inhibiting gluconeogenesis and ameliorating pancreatic damage (Ahmed et al., 2012; Mahmoud et al., 2015). Insulin can decrease blood glucose levels through stimulating glucose uptake by skeletal muscles and adipose tissue (Ramnanan et al., 2012; Newsholme and Dimitriadis, 2001).
Concerning the current results, testicular histopathological examination of DM group showed marked thickening of the tunica albuginea and the basal lamina, and widely separated seminiferous tubules with a significant decrease in their tubular diameter. Severe loss of the germinal epithelium with abnormal arrangement and morphology of the remaining cells, widening of the tubular lumen and severe decrease in the number of spermatozoa in the tubular lumen were also seen. Damage to Leydig cells was observed. Interstitial tissues showed signs of inflammation in the form of edema, hemorrhage and dilated thick-walled blood vessels. Morphometric measurements of the testicular sections of the DM group confirmed the histopathological changes. Hesperidin administration markedly restored the structure of the testicular tissues and improved the morphometric measurements of the seminiferous tubules in diabetic rats. Insulin treatment caused mild improvement of the testicular architecture and significant improvement of the morphometric measurements. These alterations seen in the DM group could be explained by hyperglycemia which increase of cellular oxidative stress due to the overproduction of ROS and decreasing the antioxidant mechanisms. These high levels of ROS had a direct damaging effect on the tubular epithelium and Leydig cells, markedly increasing their apoptosis and atrophy, and consequently affecting sperm quantity and function (Kianifard et al., 2011; Alves et al., 2013). The diabetes-related hyperinsulinemia decreases LH levels, together with the destruction of Leydig cells, cause a decrease in the androgen biosynthesis and a decrease in the serum testosterone levels leading to a delayed abnormal spermatogenesis and a reduction in sperm output and fertility. The decreased tubular diameter is due to the atrophy of the germinal epithelium and the pressure caused by the surrounding interstitial edema and hemorrhage. The inflammatory process occurring in testis is due to the occurring oxidative stress and apoptosis, causing destruction of blood vessels, edema and hemorrhage (Ballester et al., 2004). Thick tunica albuginea and tubular basal lamina were explained by either increased collagen content, due to dysfunction of fibroblasts or glycation of the collagen. This thickness impairs blood supply to the testicular cells, thus increasing their damage and atrophy (Kianifard et al., 2011). The effects of hesperidin were attributed to its capacity to ameliorate the toxic effects of DM through its powerful antioxidant, hypoglycemic action, down-regulation of the pro-inflammatory cytokines, its beneficial effects on the capillary permeability and blood flow, it reduces diabetes-induced inflammatory edema, hemorrhage and congested dilated blood vessels (Belhan et al., 2017). Insulin was unable to reverse the oxidative process and apoptosis in DM as it lacks the antioxidant/anti-apoptotic actions (Dkhil et al., 2016).
In the present study, PCNA-positive cells in the testicular tissues of the diabetic rats ranged from few cells in some seminiferous tubules to complete absence of positive cells in other tubules. The administration of hesperidin increased expression of PCNA-positive cells compared to DM group. The insulin-treated rats showed mild improvement in comparison to the DM group, but less than the hesperidin-treated group. The ability of hesperidin to stimulate the proliferation of cells in DM is attributed to its antioxidant/hypoglycemic, leading to enhanced cellular proliferation and survival stated by (Mahmoud and Hussein, 2014; Dkhil et al., 2016).
In this study, Bcl-2 sections of diabetic rats showed a marked decrease in the expression of the anti- apoptotic protein Bcl-2. Hesperidin markedly upregulated expression of Bcl-2 in the testicular tissues. Insulin was noticed to mildly increase Bcl-2 expression. This can be explained by increased testicular cell apoptosis accompanying DM (Jiang et al., 2015). Hesperidin provide evidence for the anti-apoptotic properties.
In the current work, sperm morphological abnormalities were increased significantly in the diabetic rats, in which tail abnormalities were the most common, followed by head abnormalities, then multiple abnormalities. Neck abnormalities were the least common. Administration of hesperidin to the diabetic rats greatly improved sperm abnormalities. Insulin treatment also lessened many sperm abnormalities. Increased abnormal sperm forms in the diabetic rats is attributed to disrupted glucose metabolism and the damaging effect of ROS to the spermatozoa. The susceptibility of the spermatozoa to ROS is high, particularly in the epididymis, because spermatozoa produced in the testis are reasonably more protected by the microenvironment of SCs, but they are less protected against oxidation in the epididymis, where they are stored. Oxidative stress led to DNA damage of the sperm cells, altered membrane functions, impaired motility, and decreased fertilization capacity. As insulin cannot work as an antioxidant, some abnormalities were still seen with insulin treatment.
Conclusion
Treatment with hesperidin appeared to be more effective in counteracting the toxic effects of diabetes on testes than insulin.
Author contributions
All authors have been personally, equally and actively involved in substantive work leading to the manuscript and will hold themselves jointly and individually responsible for its content.
Related articles
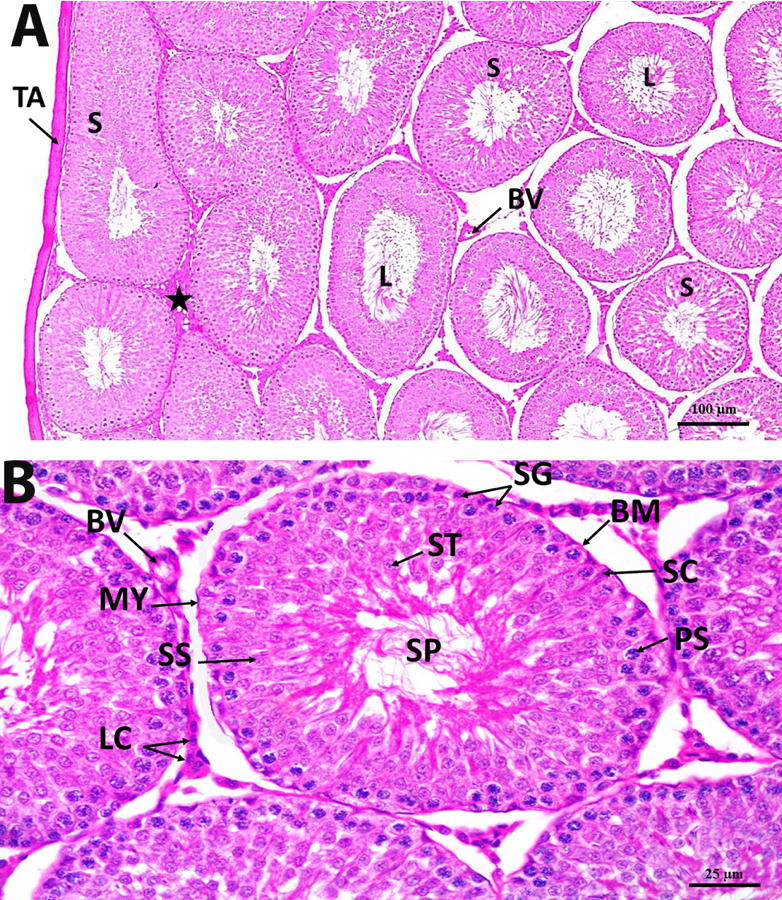 Fig. 1. Photomicrograph section in control rat testis group showing (A) homogenous tunica albuginea of normal thickness (TA), closely packed seminiferous tubules (S), germinal epithelium of normal height, lumen (L) full of spermatozoa, Normal interstitium («) containing Leydig cells and normal blood vessels (BV)(H&E x 100). (B) normal different cells of the germinal epithelium, Spermatogonia (SG), 1ry spermatocytes (PS), 2ry spermatocytes (SS), spermatids (ST) and spermatozoa (SP). Normal sertoli cells (SC) are observed between the germinal cells. The seminiferous tubules are surrounded with a basement membrane of a normal thickness (BM) and myoid cells (MY). Interstitium shows clusters of Leydig cells (LC) and normal blood vessels (BV) (H&E x 400).
Fig. 1. Photomicrograph section in control rat testis group showing (A) homogenous tunica albuginea of normal thickness (TA), closely packed seminiferous tubules (S), germinal epithelium of normal height, lumen (L) full of spermatozoa, Normal interstitium («) containing Leydig cells and normal blood vessels (BV)(H&E x 100). (B) normal different cells of the germinal epithelium, Spermatogonia (SG), 1ry spermatocytes (PS), 2ry spermatocytes (SS), spermatids (ST) and spermatozoa (SP). Normal sertoli cells (SC) are observed between the germinal cells. The seminiferous tubules are surrounded with a basement membrane of a normal thickness (BM) and myoid cells (MY). Interstitium shows clusters of Leydig cells (LC) and normal blood vessels (BV) (H&E x 400).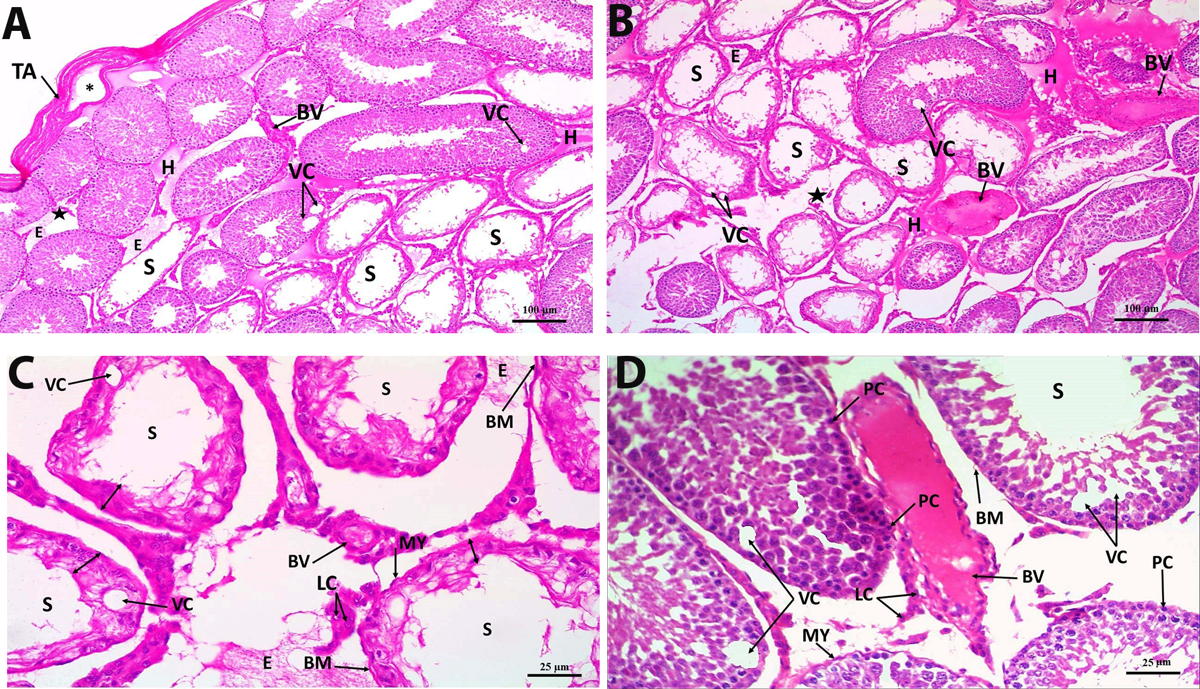 Fig. 2. Photomicrograph section in DM rat testis showing (A& B) thick tunica albuginea (TA) with cystic degeneration (*). Many seminiferous tubules show atrophied germinal epithelium (S), vacuolization (VC), lumen devoid of spermatozoa, Edema (E) and hemorrhage (H) are seen widely separating the tubules. Decreased interstitial mass is observed («), dilated and congested thick-walled blood vessels (BV) (H&E x 100). (C) seminiferous tubules (S) having almost completely atrophied germinal epithelium (double-headed arrow), vacuolization (VC) and a lumen devoid of spermatozoa. The tubules are surrounded with detached thick basement membrane (BM) and myoid cells (MY). Leydig cells are distorted (LC), Edema (E), dilated congested thick-walled blood vessels (BV) (H&E x 400). (D) seminiferous tubule (S) with a lumen devoid of spermatozoa. The epithelium of other tubules shows pyknotic cells (PC). Vacuolization (VC) is found within the germinal epithelium. Detached basement membrane (BM) and myoid cells (MY) are seen surrounding the seminiferous tubules. Leydig cells are distorted (LC). Dilated congested, thick-walled blood vessel (BV) (H&E x 400).
Fig. 2. Photomicrograph section in DM rat testis showing (A& B) thick tunica albuginea (TA) with cystic degeneration (*). Many seminiferous tubules show atrophied germinal epithelium (S), vacuolization (VC), lumen devoid of spermatozoa, Edema (E) and hemorrhage (H) are seen widely separating the tubules. Decreased interstitial mass is observed («), dilated and congested thick-walled blood vessels (BV) (H&E x 100). (C) seminiferous tubules (S) having almost completely atrophied germinal epithelium (double-headed arrow), vacuolization (VC) and a lumen devoid of spermatozoa. The tubules are surrounded with detached thick basement membrane (BM) and myoid cells (MY). Leydig cells are distorted (LC), Edema (E), dilated congested thick-walled blood vessels (BV) (H&E x 400). (D) seminiferous tubule (S) with a lumen devoid of spermatozoa. The epithelium of other tubules shows pyknotic cells (PC). Vacuolization (VC) is found within the germinal epithelium. Detached basement membrane (BM) and myoid cells (MY) are seen surrounding the seminiferous tubules. Leydig cells are distorted (LC). Dilated congested, thick-walled blood vessel (BV) (H&E x 400).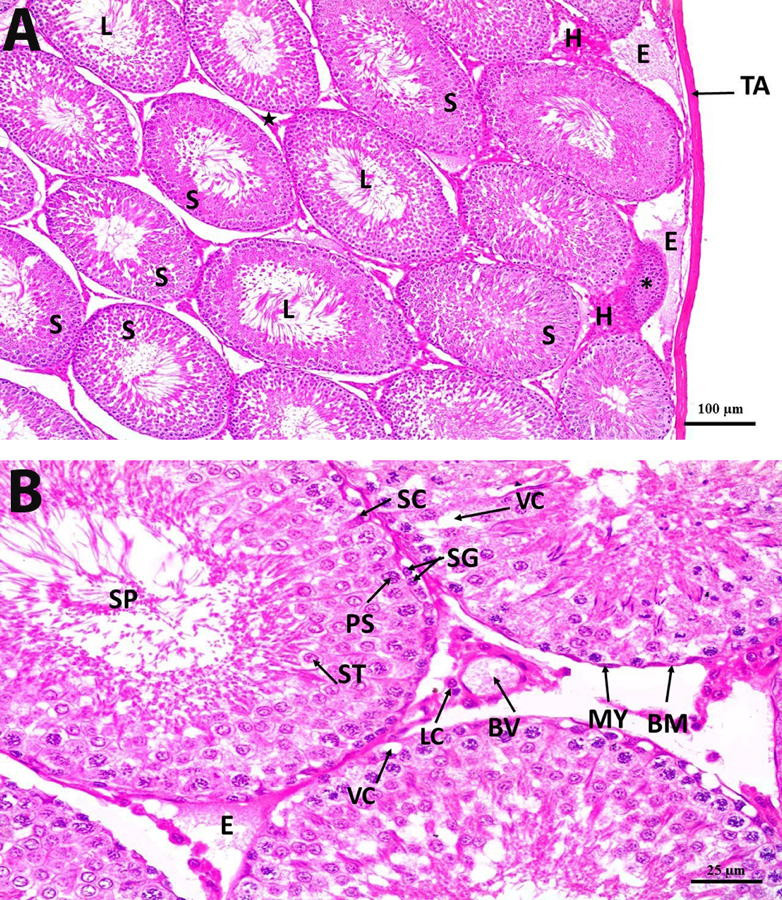 Fig. 3. Photomicrograph section in DM+H rat testis showing (A) homogenous tunica albuginea of an almost normal thickness (TA), closely packed seminiferous tubules (S) with regeneration of their germinal epithelium. The tubular lumen (L) is filled with spermatozoa. Degenerated seminiferous tubule is observed (*). Increase in the interstitial mass, containing Leydig cells, («), edema (E) and hemorrhage (H) are still observed (H&E x 100). (B) The seminiferous tubules are surrounded with a basement membrane of a near-normal thickness (BM) and lined with almost regular germinal epithelium containing different types of cells. Spermatogonia (SG), 1ry spermatocytes (PS), spermatid (ST), spermatozoa (SP) and Sertoli cells (SC). Myoid cells are also seen surrounding the tubules (MY). Vacuolization (VC) is still found within the seminiferous tubules. Interstitium shows normal Leydig cells (LC), edema (E) and thin-walled dilated blood vessels (BV) (H&E x 400).
Fig. 3. Photomicrograph section in DM+H rat testis showing (A) homogenous tunica albuginea of an almost normal thickness (TA), closely packed seminiferous tubules (S) with regeneration of their germinal epithelium. The tubular lumen (L) is filled with spermatozoa. Degenerated seminiferous tubule is observed (*). Increase in the interstitial mass, containing Leydig cells, («), edema (E) and hemorrhage (H) are still observed (H&E x 100). (B) The seminiferous tubules are surrounded with a basement membrane of a near-normal thickness (BM) and lined with almost regular germinal epithelium containing different types of cells. Spermatogonia (SG), 1ry spermatocytes (PS), spermatid (ST), spermatozoa (SP) and Sertoli cells (SC). Myoid cells are also seen surrounding the tubules (MY). Vacuolization (VC) is still found within the seminiferous tubules. Interstitium shows normal Leydig cells (LC), edema (E) and thin-walled dilated blood vessels (BV) (H&E x 400).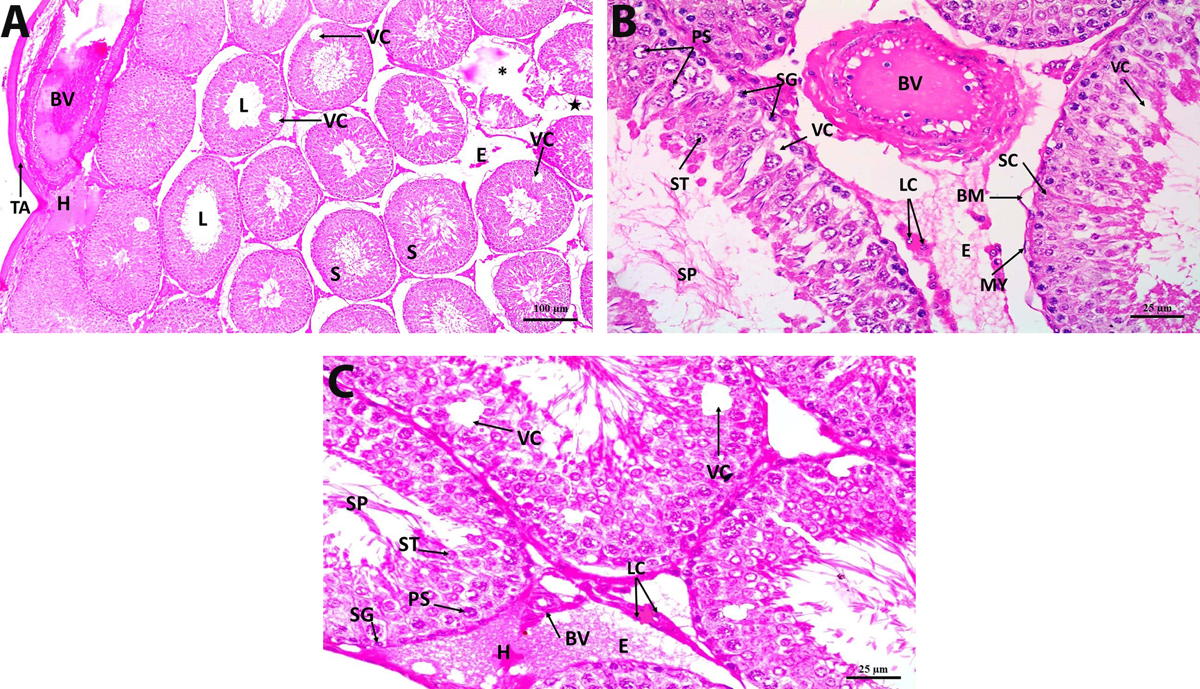 Fig. 4. Photomicrograph section in DM+In rat testis showing (A) thick tunica albuginea (TA), some seminiferous tubules appear normal (S), other tubules show vacuolization (VC), a lumen devoid of spermatozoa (L) and are degenerated (*). Distorted interstitial cells («), hemorrhage (H), edema (E) are still observed, dilated, congested thick-walled blood vessels appear in the tunica vasculosa (BV) (H&E x 100). (B) disorganized germinal epithelial cells lining the seminiferous tubules. Spermatogonia (SG), 1ry spermatocytes (PS), spermatids (ST) and spermatozoa (SP) are seen. Sertoli cells (SC) are occasionally seen within the disrupted germinal cells. Vacuolization (VC) is still found within the seminiferous tubules. The tubules are surrounded with detached basement membrane (BM). Myoid cells (MY). Interstitium shows abnormal Leydig cells (LC), edema (E) and dilated, congested thick-walled blood vessels (BV) (H&E x 400). (C) disorganized germinal epithelial cells lining the seminiferous tubules. Spermatogonia (SG), 1ry spermatocytes (PS), spermatids (ST) and spermatozoa (SP) are seen. Vacuolization (VC) is still found within the seminiferous tubules. Interstitium shows some regeneration of Leydig cells (LC), edema (E), hemorrhage (H) and thick-walled blood vessels (BV) (H&E x 400).
Fig. 4. Photomicrograph section in DM+In rat testis showing (A) thick tunica albuginea (TA), some seminiferous tubules appear normal (S), other tubules show vacuolization (VC), a lumen devoid of spermatozoa (L) and are degenerated (*). Distorted interstitial cells («), hemorrhage (H), edema (E) are still observed, dilated, congested thick-walled blood vessels appear in the tunica vasculosa (BV) (H&E x 100). (B) disorganized germinal epithelial cells lining the seminiferous tubules. Spermatogonia (SG), 1ry spermatocytes (PS), spermatids (ST) and spermatozoa (SP) are seen. Sertoli cells (SC) are occasionally seen within the disrupted germinal cells. Vacuolization (VC) is still found within the seminiferous tubules. The tubules are surrounded with detached basement membrane (BM). Myoid cells (MY). Interstitium shows abnormal Leydig cells (LC), edema (E) and dilated, congested thick-walled blood vessels (BV) (H&E x 400). (C) disorganized germinal epithelial cells lining the seminiferous tubules. Spermatogonia (SG), 1ry spermatocytes (PS), spermatids (ST) and spermatozoa (SP) are seen. Vacuolization (VC) is still found within the seminiferous tubules. Interstitium shows some regeneration of Leydig cells (LC), edema (E), hemorrhage (H) and thick-walled blood vessels (BV) (H&E x 400). Fig. 5. A photomicrograph of a section of the rat seminiferous tubules with PCNA immuno-stain showing (A) control group: brown PCNA-positive nuclei that are markedly detected in the spermatogonia and early-stage spermatocytes. (B) DM group: marked decrease in the expression of brown PCNA-positive nuclei. (C) DM+H group: moderate expression of brown PCNA-positive nuclei. (D) DM+In group: mild expression of brown PCNA-positive nuclei (PCNA immuno-stain x 400).
Fig. 5. A photomicrograph of a section of the rat seminiferous tubules with PCNA immuno-stain showing (A) control group: brown PCNA-positive nuclei that are markedly detected in the spermatogonia and early-stage spermatocytes. (B) DM group: marked decrease in the expression of brown PCNA-positive nuclei. (C) DM+H group: moderate expression of brown PCNA-positive nuclei. (D) DM+In group: mild expression of brown PCNA-positive nuclei (PCNA immuno-stain x 400).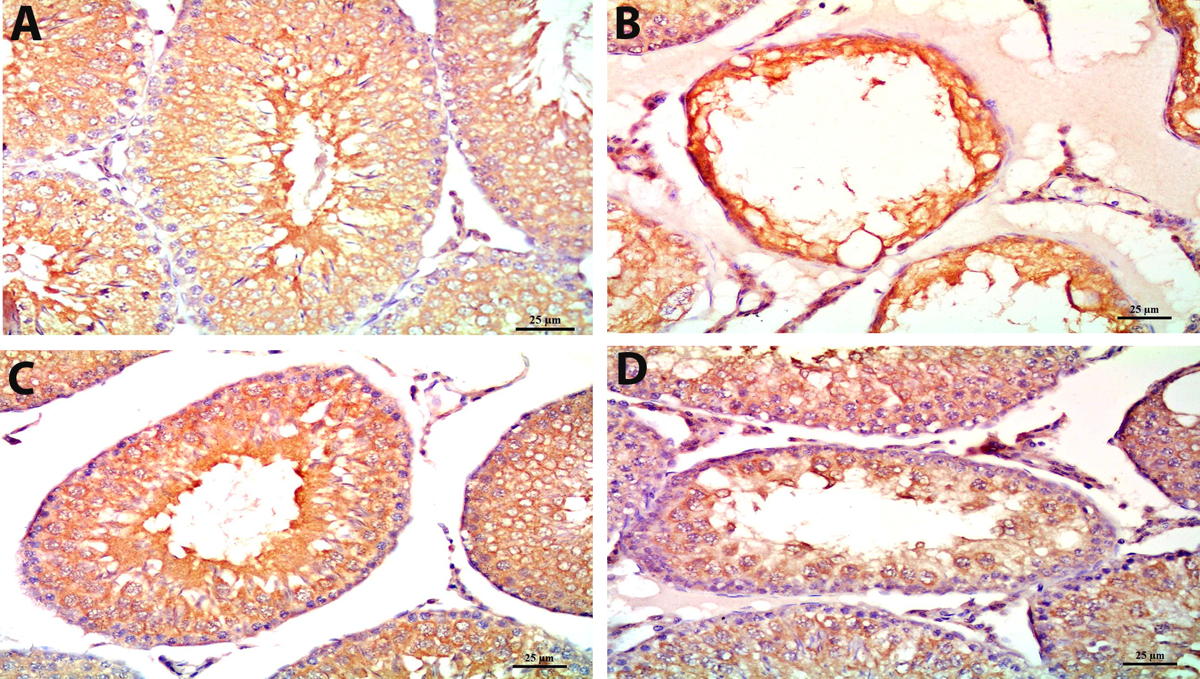 Fig. 6. A photomicrograph of a section of the rat seminiferous tubules with Bcl-2 immuno-stain showing (A) control group: marked expression of brown Bcl-2 protein in most tubular cells, mainly near the tubular lumen. (B) DM group: marked decrease in the expression of brown Bcl- 2 protein in the tubular cells. (C) DM+H group: moderate expression of brown Bcl-2 protein in the tubular cells. (D) DM+In group: mild expression of brown Bcl-2 protein in the tubular cells (Bcl-2 immuno-stain x 400).
Fig. 6. A photomicrograph of a section of the rat seminiferous tubules with Bcl-2 immuno-stain showing (A) control group: marked expression of brown Bcl-2 protein in most tubular cells, mainly near the tubular lumen. (B) DM group: marked decrease in the expression of brown Bcl- 2 protein in the tubular cells. (C) DM+H group: moderate expression of brown Bcl-2 protein in the tubular cells. (D) DM+In group: mild expression of brown Bcl-2 protein in the tubular cells (Bcl-2 immuno-stain x 400).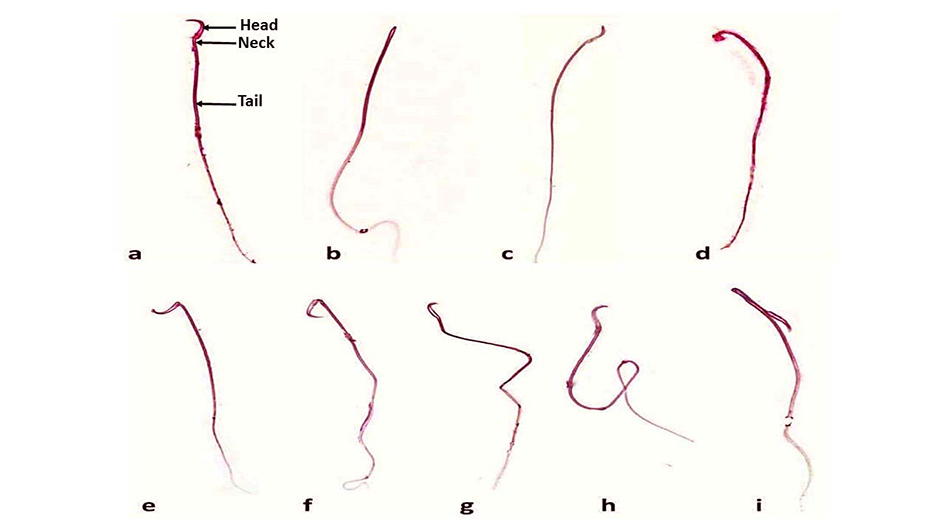 Fig. 7. A photomicrograph illustrating sperm morphology. a: normal (showing head, neck and tail), b: absent head abnormality, c: hook-less head abnormality, d: amorphous head abnormality, e: abnormal backward bent of the neck, f: abnormal forward bent of the neck, g: bent tail abnormality, h: ring tail abnormality and i: multiple abnormalities in a single sperm (note the hook-less head and the bent tail) (Eosin x 400).
Fig. 7. A photomicrograph illustrating sperm morphology. a: normal (showing head, neck and tail), b: absent head abnormality, c: hook-less head abnormality, d: amorphous head abnormality, e: abnormal backward bent of the neck, f: abnormal forward bent of the neck, g: bent tail abnormality, h: ring tail abnormality and i: multiple abnormalities in a single sperm (note the hook-less head and the bent tail) (Eosin x 400).ACIPAYAM C, BAYRAM I, DAGLIOGLU K, DORAN F, YILMAZ S, SEZGIN G, TOTAN ATEŞ B, OZKAN A, TANYELI A (2014) The protective effect of hesperidin on methotrexate-induced intestinal epithelial damage in rats: an experimental study. Medical Principles and Practice, 23(1): 45-52.
AHMED OM, MAHMOUD AM, ABDEL-MONEIM A, ASHOUR MB (2012) Antidiabetic effects of hesperidin and naringin in type 2 diabetic rats. Diabetologia Croatica, 41(2): 53-67.
ALLUANAN ADELSON DO NASCIMENTO S, JESSICA SANTANA DE O, RODRIGO RIBEIRO DE O, SÍLVIA REGINA ARRUDA DE M, VALDEMIRO AMARO DA SILVA J, ELIZABETH NEVES DE M (2014) Evaluation of quantitative parameters of Leydig cell in diabetic adults rats. Acta Scientiarum Biol Sci, 36(4): 483-489.
ALVES MG, MARTINS AD, RATO L, MOREIRA PI, SOCORRO S, OLIVEIRA PF (2013) Molecular mechanisms beyond glucose transport in diabetes-related male infertility. Biochim Biophys Acta - Molecular Basis of Disease, 1832(5): 626-635.
ARAFA H, ALY H, ABD-ELLAH M, EL-REFAEY H (2009) Hesperidin attenuates benzo[α] pyrene-induced testicular toxicity in rats via regulation of oxidant/antioxidant balance. Toxicol Industrial Health 25(6): 417-427.
BALLESTER J, MUÑOZ MC, DOMÍNGUEZ J, RIGAU T, GUINOVART JJ, RODRÍGUEZ-GIL JE (2004) Insulin-dependent diabetes affects testicular function by FSH- and LH-linked mechanisms. J Androl, 25(5): 706-719.
BELHAN S, ÖZKARACA M, KANDEMİR FM, GÜLYÜZ F, YILDIRIM S, ÖMÜR AD, YENER Z (2017) Effectiveness of hesperidin on methotrexate-induced testicular toxicity in rats. Kafkas Üniversitesi Veteriner Fakültesi Dergisi, 23(5): 789-796.
CHENG D, LIANG B, LI Y (2013) Antihyperglycemic effect of Ginkgo biloba extract in streptozotocin-induced diabetes in rats. Biomed Res Int, 2013: 162724.
DKHIL MA, ZRIEQ R, AL-QURAISHY S, ABDEL MONEIM AE (2016) Selenium nanoparticles attenuate oxidative stress and testicular damage in streptozotocin-induced diabetic rats. Molecules, 21(11): 1517.
EBONG PE, EFIONG EE, MGBEJE BI, IGILE GO, ITAM EH (2014) Combined therapy of Moringa oleifera and Ocimum gratissimum reversed testicular damage in diabetic rats. J Adv Med Medical Res, 4(11): 2277-2290.
ELSHAZLY SM, ABD EL MOTTELEB DM, IBRAHIM IAAEH (2018) Hesperidin protects against stress induced gastric ulcer through regulation of peroxisome proliferator activator receptor gamma in diabetic rats. Chemico-Biological Interactions, 291: 153-161.
GHLISSI Z, HAMDEN K, SAOUDI M, SAHNOUN Z, ZEGHAL KM, EL FEKI A, HAKIM A (2012) Effect of Nigella sativa seeds on reproductive system of male diabetic rats. Afr J Pharm Pharmacol, 6 (20): 1444-1450.
IDRIS MHM, BUDIN SB, OSMAN M, MOHAMED J (2012) Protective role of Hibiscus sabdariffa calyx extract against streptozotocin induced sperm damage in diabetic rats. Excli Journal, 11: 659- 669.
JAIN G, JANGIR R (2014) Modulation of diabetes-mellitus-induced male reproductive dysfunctions in experimental animal models with medicinal plants. Pharmacognosy Rev, 8: 113-121.
JASMIN, JAITAK V (2019) A review on molecular mechanism of flavonoids as antidiabetic agents. Mini Reviews Med Chem, 19 (9): 762-786.
JIANG X, CHEN J, ZHANG C, ZHANG Z, TAN Y, FENG W, SKIBBA M, XIN Y, CAI L (2015) The protective effect of FGF21 on diabetes-induced male germ cell apoptosis is associated with up-regulated testicular AKT and AMPK/Sirt1/PGC-1α signaling. Endocrinology, 156(3): 1156-1170.
KALPANA KB, SRINIVASAN M, MENON VP (2009) Evaluation of antioxidant activity of hesperidin and its protective effect on H2O2 induced oxidative damage on pBR322 DNA and RBC cellular membrane. Mol Cell Biochem, 323(1): 21-29.
KANTER M, AKTAS C, ERBOGA M (2012) Protective effects of quercetin against apoptosis and oxidative stress in streptozotocin-induced diabetic rat testis. Food Chemical Toxicol, 50(3-4): 719-725.
KASSAB BM, HUSSEIN HH, MAHMOUD OM, ABDEL-ALRAHMAN G (2019) Effects of insulin and metformin on fetal kidney development of streptozotocin-induced gestational diabetic albino rats. Anat Cell Biol, 52(2): 161-175.
KAYA K, CIFTCI O, CETIN A, DOĞAN H, BAŞAK N (2015) Hesperidin protects testicular and spermatological damages induced by cisplatin in rats. Andrologia, 47(7): 793-800.
KIANIFARD D, SADRKHANLOU RA, HASANZADEH S (2011) The histological, histomorphometrical and histochemical changes of testicular tissue in the metformin treated and untreated streptozotocin- induced adult diabetic rats. Vet Res Forum, 2 (1): 13-24.
LATIF R, LODHI GM, ASLAM M (2008) Effects of amlodipine on serum testosterone, testicular weight and gonado-somatic index in adult rats. J Ayub Med Coll, Abbottabad JAMC, 20 (4): 8-10.
LONG L, QIU H, CAI B, CHEN N, LU X, ZHENG S, YE X, LI Y (2018) Hyperglycemia induced testicular damage in type 2 diabetes mellitus rats exhibiting microcirculation impairments associated with vascular endothelial growth factor decreased via PI3K/Akt pathway. Oncotarget, 9 (4): 5321-5336.
MAHMOUD AM, HUSSEIN OE (2014) Hesperidin as a promising anti-diabetic flavonoid: the underlying molecular mechanism. Int J Food Nutr Sci, 3 (3): 1.
MAHMOUD AM, AHMED OM, ASHOUR MB, ABDEL-MONEIM A (2015) In vivo and in vitro antidiabetic effects of citrus flavonoids; a study on the mechanism of action. Int J Diabetes Dev Countries, 35 (3): 250-263.
MAHMOUD OM, AL BADAWI MH, SALEM NA (2014) Role of Ginseng on mercury chloride-induced testicular lesions in adult albino rat: a histological and immunohistochemical study. Egypt J Histol, 37 (3): 506-513.
MOIR AMB, ZAMMIT VA (1994) Effects of insulin treatment of diabetic rats on hepatic partitioning of fatty acids between oxidation and esterification, phospholipid and acylglycerol synthesis, and on the fractional rate of secretion of triacylglycerol in vivo. Biochem J, 304 (1): 177-182.
NEWSHOLME EA, DIMITRIADIS G (2001) Integration of biochemical and physiologic effects of insulin on glucose metabolism. Exp Clin Endocrinol Diabetes, 109 (Suppl 2): S122-S134.
OLDEREID NB, ANGELIS PD, WIGER R, CLAUSEN OP (2001) Expression of Bcl-2 family proteins and spontaneous apoptosis in normal human testis. Mol Human Reprod, 7 (5): 403-408.
RAMALHO-SANTOS J, AMARAL S, OLIVEIRA PJ (2008) Diabetes and the impairment of reproductive function: possible role of mitochondria and reactive oxygen species. Current Diabetes Rev, 4 (1): 46-54.
RAMNANAN CHRISTOPHER J, EDGERTON DALE S, CHERRINGTON ALAN D (2012) Evidence against a physiologic role for acute changes in CNS insulin action in the rapid regulation of hepatic glucose production. Cell Metab, 15 (5): 656-664.
RICCI G, CATIZONE A, ESPOSITO R, PISANTI FA, VIETRI MT, GALDIERI M (2009) Diabetic rat testes: morphological and functional alterations. Andrologia, 41 (6): 361-368.
SANGAMESWARAN B, JAYAKAR B (2008) Anti-diabetic, anti-hyperlipidemic and spermatogenic effects of Amaranthus spinosus Linn. on streptozotocin-induced diabetic rats. J Nat Med, 62 (1): 79-82.
SCHILLER AB, CLARK WS, COTSONIS G, LAWSON D, DEROSE PB, COHEN C (2002) Image cytometric bcl-2:bax and bcl-2:bcl-x ratios in invasive breast carcinoma: correlation with prognosis. Cytometry, 50 (4): 203-209.
SCHNEIDER CA, RASBAND WS, ELICEIRI KW (2012) NIH Image to ImageJ: 25 years of image analysis. Nature Methods, 9 (7): 671-675.
SCHOELLER EL, ALBANNA G, FROLOVA AI, MOLEY KH (2012) Insulin rescues impaired spermatogenesis via the hypothalamic-pituitary-gonadal axis in Akita diabetic mice and restores male fertility. Diabetes, 61 (7): 1869.
SUDHA S, VALLI G, JULIE PM, ARUNAKARAN J, GOVINDARAJULU P, BALASUBRAMANIAN K (2000) Influence of streptozotocin-induced diabetes and insulin treatment on the pituitarv-testicular axis during sexual maturation in rats. Exp Clin Endocrinol Diabetes, 108 (01): 14-20.
SURESH S, PRITHIVIRAJ E, PRAKASH S (2010) Effect of Mucuna pruriens on oxidative stress mediated damage in aged rat sperm. Int J Androl, 33 (1): 22-32.
TROMMELEN J, GROEN B, HAMER HM, GROOT CPGMD, LOON LJC (2015) Exogenous insulin does not increase muscle protein synthesis rate when administered systemically: a systematic review. Eur J Endocrinol, 173: R25-R34.
UGARTE M, BROWN M, HOLLYWOOD KA, COOPER GJ, BISHOP PN, DUNN WB (2012) Metabolomic analysis of rat serum in streptozotocin-induced diabetes and after treatment with oral triethylenetetramine (TETA). Genome Medicine, 4 (4): 35.
VIJAYA BHARATHI B, JAYA PRAKASH G, KRISHNA KM, RAVI KRISHNA CH, SIVANARAYANA T, MADAN K, RAMA RAJU GA, ANNAPURNA A (2015) Protective effect of alpha glucosyl hesperidin (G-hesperidin) on chronic vanadium induced testicular toxicity and sperm nuclear DNA damage in male Sprague-Dawley rats. Andrologia, 47 (5): 568-578.
VISNAGRI A, KANDHARE AD, CHAKRAVARTY S, GHOSH P, BODHANKAR SL (2014) Hesperidin, a flavanoglycone attenuates experimental diabetic neuropathy via modulation of cellular and biochemical marker to improve nerve functions. Pharmaceutical Biol, 52 (7): 814-828.