The objective of this study was to analyze the potential hepatotoxicity of the doxorubicin (DOX). 50 male albino rats were treated with doxorubicin daily for 30 days. Hepatotoxicity was monitored by quantitative analysis of the serum alanine aminotransferase (ALT), Gamma-glutamyl transferase (γ-GT) activities, total protein, and albumin. The second aim of this study to investigate affected chlorpyrifose or glyphosate alone or together on lipid profile levels of cholesterol, triglyceride, low-density lipoprotein-cholesterol (LDL-C) and high-density lipoprotein-cholesterol (HDL-C) respectively, Triiodothyronine (T3), thyroxin (T4) and thyroid-stimulating hormone (TSH) were measured, and livers were collected for histopathological study for light and electron microscope. The results testify to significant elevation liver functions (ALT and GGT), as well as significant decrease in total protein and albumin level. The hormones registered a significant decrease in T3 and T4 while there is an increase in TSH. Histopathology revealed vacuolar of hepatocytes with random hepatocyte necrosis and mononuclear cell infiltration. The administration of the MSCs and placenta extract have beneficial and decrease side-effects against the deleterious changes of Doxorubicin. Histopathology revealed degeneration vacuolar of hepatocytes with random hepatocyte necrosis and mononuclear cell infiltration. The administration of the MSCs and HPL had beneficial and decrease side effects against the deleterious changes of DOX. In conclusion, results suggest a potential contribution of DOX to the etiology of some diseases, while MSCs and PE have beneficial effects, as they tends to dampen DOX toxicity in rats.
Histological and biochemical alterations in the livers of rats treated with MSCs and placental extract against Doxorubicin as chemotherapy
Alsayed A. Abdelhady1, Mahmoud. Diab2, Ahmed Nabeeh3
1 Anatomy and Embryology Department, Faculty of Medicine, Helwan University Cairo, Egypt
2 Anatomy and Embryology Department, Faculty of Medicine. Al-Azhar University Cairo, Egypt
3 Zoology Department, Faculty of Science, Al-Azhar University Cairo, Egypt
SUMMARY
Sign up or Login
INTRODUCTION
Chemotherapy employs chemical agents to discontinue the growth and annihilate cancer cells even at remote sites from the source of the primary tumor. However, it does not discriminate between cancer and normal cells, and eradicates not only fast-growing cancer cells but also other rapidly growing cells in the body. Chemotherapeutic drugs used to treat cancer are given to most of the people which help to sustain the completion of cancer treatment (El-Sayyad et al., 2009). In addition to devastating production of reactive oxygen species (ROS) the destructive side effects of chemotherapeutic agents have to be considered. Therefore, an escalating amount of facts suggests that the simultaneous treatment of chemotherapy and chemo-preventive agents with antioxidant action may augment the efficacy of chemotherapeutics (Aydin et al., 2011). It is very active against a wide spectrum of cancers, and is mainly used in the treatment of lymphomas, leukemia and other solid tumors like carcinoma of ovaries, breast, lung, thyroid, etc. (Gianni et al., 2007). Doxorubicin (DOX) is an anthracycline glycoside antibiotic that acquires an effective and broad-spectrum antitumor activity against a variety of human solid tumors like ovarian, breast, lung, uterine and cervical cancers, Hodgkin’s disease, soft tissue, and primary bone sarcomas, as well against several other cancer types and hematological malignancies (Chang et al., 2011; Thippeswamy et al., 2011). However, its use in chemotherapy has been restricted mostly due to its varied toxicities including cardiac, pulmonary, hepatic, renal, hematological and testicular toxicity (Mohan et al., 2010). Doxorubicin as an anticancer agent can cause dose-dependent cardiotoxicity and heart failure in the long term. Rutin is a polyphenolic flavonoid that has been proved to protect hearts from diverse cardiovascular diseases (Ma et al., 2017). Doxorubicin (DOX), a prominent anticancer agent, has enjoyed considerable popularity in the last few decades because of its usefulness in the management of various forms of cancers, but its organotoxic potential (cardiotoxicity, hepatotoxicity, and nephrotoxicity) has constrained its clinical use (Lahoti et al., 2012). Hepatotoxicity is one of the main side effects associated with Doxorubicin (DOX) treatment (Mohan et al., 2011). Multipotent MSCs (mesenchymal stem cells) have shown potential in tissue regeneration in human diseases (Salem and Thiemermann, 2010). MSCs represent a rare heterogeneous subset of pluripotent stromal cells that can be isolated from a number of different adult tissues as well as BM (bone marrow), and have the potential to give rise to cells of diverse lineages. Thus, MSCs are an attractive cell source for regenerative medicine. Numerous studies have reported the beneficial effects of MSCs in tissue repair and regeneration (Quertainmont et al., 2012; Kon et al., 2012; Joyce et al., 2012). After culture expansion and in vivo administration, MSCs home and engraft in injured tissue and modulate the inflammatory response through synergistic downregulation of pro-inflammatory cytokines and up-regulation of pro-survival and anti-inflammatory factors (Cho et al., 2012). Besides their differentiating potentials, autologous bone-marrow-derived mesenchymal stem cells (BMSCs) can be isolated from the bone marrow and expanded, which makes BMSCs a conceivable source of stem cells for repairing damaged tissues. So far, BMSCs have been tested in several animal brain and heart ischemia models and have shown beneficial effects by promoting tissue repair and functional recovery (Hu et al., 2008). Placenta embedding therapy began in the 1930s. The extraction of active ingredients from the human placenta was established in the 1960s, and human placental extract (HPE) was later approved by the Food and Drug Administration for use in humans (Kwon et al., 2015). The human placenta is an organ for fetus development and an abundant reservoir of various bioactive molecules. Interest in human placenta extract (HPE) is growing, and application with a trial of HPE is widening in oriental medicine, including liver diseases (Jung et al., 2011). Human placental extract (HPE) is a source of numerous biologically active molecules and has been used clinically to treat chronic hepatitis, liver cirrhosis and other chronic diseases (Yamauchi et al., 2019). Studies using animal models have provided evidence that placenta extract improves liver function (Jung et al., 2011), and wound healing (Hong et al., 2010). In clinical situations, HPE has been prescribed to treat chronic hepatitis, liver cirrhosis, viral hepatitis, and other hepatic diseases. HPE is also used in the treatment of menopausal symptoms (Kong et al., 2008; Wu et al., 2008). Analysis of human placenta extracts (HPEs) has revealed that such extracts appear to possess antioxidant activity. Thus, HPEs have been shown to scavenge hydroxyl radical, nitric oxide and superoxide radical; to reduce ferric iron; to chelate transition metal ions; and to prevent lipid peroxidation (Rozanova et al., 2010).
MATERIALS AND METHODS
Animals
All animals in this study were conducted under the criteria of the investigations and Ethics Committee of the Community Laws governing the use of experimental animals.
Mature male Sprague Dawley albino rats of average weights (200-220 g) (obtained from laboratory of Schistosoma Biological Supply Program -SBSP- Theodor Bilharz Research Institute) were housed in stainless steel cages with water and food ad libitum, temperature of 22±2ºC, humidity around 56% and 12 h light-dark cycle. The rats were transferred to the animal house in Zoology Department, Faculty of Science, Al-Azhar University, Cairo.
Chemicals and reagents
Doxorubicin (DOX) dose used in this study to induce hepatotoxicity was 5 mg/kg/week for 4 weeks (Oliveira et al., 2013). Mesenchymal Stem Cells Dose (5 × 106) in 200 µl platelets rich plasma (PRP) according to methods of Zahkouk et al. (2015). Placental extract (Laennec) used by liver cirrhosis patients for liver regeneration (2 ml/70 kg). This dose was converted to rat dose using conversion factors by Paget and Barnes (1964) to suit (0.4 ml/200 g) rats.
Mesenchymal Stem Cells (MSCs) preparation
MSCs were isolated according to a protocol modified from Snykers et al. (2006).
Placental extract dose
Placental extract (Laennec) used by liver cirrhosis patients for liver regeneration (2 ml/70 kg). This dose was converted to rat dose using conversion factors by Paget and Barnes (1964) to suit (0.4 ml/200 g) rats.
Rat BMSC cultures
Bone marrow-derived mesenchymal stem cells (BMSCs) were isolated from Wistar rats as previously described. In brief, BMSCs were obtained from the femoral and tibial bones of rats. Cells were flushed from the femurs and tibias of rats using a 25-gauge needle. Mononuclear cells were suspended in Dulbecco’s modified Eagle’s medium supplemented with 10% fetal bovine serum and plated in flasks. Cultures were maintained at 37°C in a humidified atmosphere containing 5% carbon dioxide. After 24 h, non-adherent cells were discarded, and adherent cells were washed three times with phosphate-buffered saline solution (PBS). Fresh complete medium was added and replaced every 4 days. Each primary culture was sub-cultured 1:2 when BMSCs grew to 80% confluency (Zeng et al., 2012).
Platelet-Rich Plasma (PRP) preparation
PRP preparation was carried out by adapting the protocol proposed by Sonnleitner et al. (2000).
Experimental design
50 male albino rats were randomly divided into 10 equal groups and labeled as groups 1, 2, 3, 4 and 5, each group contain 10 rats. Rats received all treatments daily via oral gavage tube along the period of the experiment. Group 1: Control rats; Group 2: 10 rats that received 4 injections of 5 mg/kg body weight (B.W) (i.p) of doxorubicin (DOX) every week. Group 3: 10 rats received DOX as the above regimen of group 2 and then left for one week without medication before being injected with MSCs therapy in a single dose of 5 x 106 in 200 ml (PRP)/ week MSCs per rat for 4 weeks via the caudal vein. Group 4: 10 rats received DOX as the above regimen of group 2 and then left for one week without medication before being injected with placental extract in a single dose of (40 µl Placental Extract) / week for 4 weeks via the caudal vein. Group 5: 10 rats received DOX as the above regimen of group 2 and then left for one week without medication before being injected with placental extract and MSCs therapy. The animals will observe daily for a sign of toxicity during the period of the experiment. The ten rats from each group scarified after the 30 days.
Sample collection
The rats were anesthetized through i.p injection of Thiopental Sodium (6 mg/kg) (Harms and Ojeda, 1974) on day 30 and blood samples were collected from all animals through retro-orbital venous plexus. Put into chilled non heparinized tubes, serum was obtained by centrifugation at 3000 r.p.m for 10 minutes; sera were frozen at -20℃ for estimation of liver functions, and hormonal profile. Animals were sacrificed after 24 hours of the last treatment, the abdominal cavities were opened, livers were rapidly and carefully excised and all attached vessels and ligaments were trimmed off to work light microscope and transmission electron microscopy (TEM).
Histopathological examination
The liver tissues were excised and immediately fixed in 10% buffered formalin at the end of the experiment. The tissue specimen was embedded in paraffin after being dehydrated in alcohol and subsequently cleared with xylene. Five-micrometer thick serial histological sections were obtained from the paraffin blocks and stained with hematoxylin and eosin (Suvarna et al., 2013). The sections were examined under light microscope to evaluate pathological changes and photomicrographs were taken.
For transmission electron microscope (TEM) examination, small liver specimens (1 mm3) were fixed in 2.5% glutaraldehyde solution. They were then post-fixed in 1% osmium tetroxide, dehydrated and embedded in Epon. Ultrathin sections were cut, stained with uranyl acetate and lead citrate (Johannessen, 1978) and then examined using TEM1010- EXII (Joel, Tokyo, Japan) at the electron microscopic unit at the Regional Center for Mycology and Biotechnology (RCMB), Al-Azhar University.
Biochemical parameters
Serum alanine aminotransferase (ALT) was determined according to the method of Bergmeyer et al. (1986). Gamma-glutamyl transferase (γ-GT) was determined according to the method of Rosalki et al. (1971). Total protein (TP) was determined according to the method described by Gornal et al. (1949). Albumin (ALB) was determined according to the method of Doumas et al. (1971). Serum triglycerides were determined according to the method described by Fossati and Prencipe (1982). Cholesterol level was determined according to the method described by Allain et al. (1974). The LDL-C calculations were conducted according to the formula of Wieland and Seidel (1982). Serum HDL-C level was determined according to the method described by Burstein et al. (1970) using the kit from Elitech diagnostic Co. France.
Hormonal profile
Serum T3 and T4 were determined according to the method described by Wheeler and Lazarus (1994). TSH was determined according to the method described by Beck (1986) and Caldwell et al. (1985). Using the electro-chemiluminescence immunoassay “ECLIA” is intended for use on Elecsys and Cobas e immunoassay analyzers.
Statistical analysis
The statistical package for social sciences SPSS/PC computer program (version 19) was used for statistical analysis of the results. Data were analyzed using one-way analysis of variance (ANOVA). The data were expressed as mean ± S.E. Differences were considered statistically significant at (P < 0.05).
RESULTS
Histopathological findings
Light microscopic findings
Group 1 - Control rats: Examined serial sections from the liver of this group revealed normal histo-morphological structures of the examined organs. Liver sections showed preserved lobular arrangement, hepatic cords orientations, portal triads structural components, sinusoids, on Kupffer cells and stroma (Fig. 1A, B).
Group 2 - Doxorubicin (anthracycline) treated rats: Liver sections revealed dramatic histopathological changes represented by moderate to massive portal and interstitial aggregations of round cells, mostly lymphocytes and plasma cells, sometime eosinophils were included. The bile ducts appeared moderately hyperplastic and suffered chronic obstructive cholangitis. Mild portal vascular congestion and perivascular edema were seen. Multifocal hepatocellular necrosis with partial replacement by inflammatory cells and erythrocytes were seen. A moderate number of hepatocytes at the vicinity of the aforementioned lesions were atrophied, apoptotic or degenerated (Fig. 1C, D).
Group 3 - Doxorubicin (anthracycline) treated rats co-administered with MSCs. Liver sections of this group revealed mild to moderate portal and interstitial aggregations of lymphocytes. Moderate numbers of hepatocytes were degenerated (hydropic degeneration) (Fig. 1E, F).
Group 4 - Doxorubicin (anthracycline) treated rats co-administered with placental extracts: Changes in this group were little pet worth. Liver sections denoted degenerative, apoptotic and necrotic changes in a moderate number of hepatocytes. Most of the degenerative changes were the hydropic type. The portal triads showed moderate aggregation of lymphocytes, plasma cells, and eosinophils. The bile ducts were mildly hyperplastic (Fig. 1G, H).
Group 5 - Doxorubicin (anthracycline) treated rats co-administered with both MSCs and PE: Changes in this group were promising as hepatic was apparently normal with preserved histomorphological of liver lobules, hepatic cord arrangement, portal triads structures, sinusoids, and Von-Kupffer cells (Fig. 1I, J).
Electron microscopic findings
Group 1: Liver of control rats showed normal nuclear structures with preserved nuclear membrane and nucleolus. The mitochondrial contents and the rough endoplasmic reticulum beside the Golgi complex appeared normal with activated functional morphology. There were no abnormal depositions, vacuoles, electron-dense bodies or phagolysosomes (Fig. 2A, B).
Group 2. Doxorubicin treated rats: All the cellular structural contents revealed different degenerative and necrotic changes. The nucleus and nuclear membrane showed pyknosis and degenerative reactions. The mitochondria revealed dilated cisterns and membranous swelling (degenerated mitochondria). A large number of rough endoplasmic reticulum appeared lost, damaged and distorted. Intracytoplasmic phagolysosomes, endosomes and vacuoles were prominent. Electron dense bodies of different shape and size (damaged ribosomes) were encountered. The glycogen storage contents of most cells were depleted (Figs. 2C, D).
Group 3. Doxorubicin-treated rats co-administrated with MSCs: electron-micrographs from this group revealed a highly activated nucleus with normal nuclear chromatin and intact nuclear membranes. The cytoplasmic organelles represented highly active and prominent mitochondria, rough endoplasmic reticulum and Golgi apparatus. Some of the mitochondria appeared elongated. The intercellular cytoplasmic membrane, intercellular bridges, and desmosomes appeared healthy (Fig. 2E, F).
Group 4. Doxorubicin-treated rats co-administrated with placental extracts: this group showed normal nucleus, nuclear chromatin and intact nuclear membranes. The cytoplasmic organelles were run in parallel with highly active and prominent mitochondria, rough and smooth endoplasmic reticulum and dispersed electron-dense ribosomes. The intercellular cytoplasmic membrane was apparently healthy. The portal triads, hepatic sinusoids, Von-Kupffer cells, and bile canaliculi were all well preserved (Fig. 2G, H).
Group 5. Doxorubicin-treated rats co-administrated with MSCs and placental extracts: A prominent regenerative activity in hepatocytes was a characteristic feature. The nuclei, nucleoli, nuclear membranes, mitochondria, rough endoplasmic reticulum, and Golgi apparatus, all, were highly activated. The cytoplasm of most cells showed moderate amounts of stored glycogen and electron-dense dispersed ribosomes. The portal area showed dilated blood spaces and the intercellular plasma membrane was prominent (Fig. 2I, J).
Biochemical results
Doxorubicin induced hepatic damage as reflected by significantly (p < 0.05) elevated serum ALT and γ-GT enzymes activities when compared to control group after 30 days. Rats treated with DOX + MSCs, DOX + placental extract (PE) and DOX + MSCs + placental extract (PE) revealed a significant decrease (p < 0.05) when compared with intoxicated groups after 30 days (Table 2).
Data presented in Table 2 recorded that a significant decrease (p < 0.05) in serum total protein (TP) and albumin (ALB) level in rats intoxicated with doxorubicin when compared with control group after 30 days. Rats treated with DOX + MSCs, DOX +PE and DOX + MSCs + PE observed a significant increase (p < 0.05) when compared with intoxicated groups after 30 days.
Doxorubicin induced hepatic damage as reflected by significantly (p < 0.05) elevated serum TC, TG and LDL-L when compared to control group after 30 days. Rats treated with DOX + MSCs, DOX + PE and DOX + MSCs + PE revealed a significant decrease (p < 0.05) when compared with intoxicated groups after 30 days (Table 2).
Data presented in Table 2 recorded a significant decrease (p < 0.05) in serum HDL-C level in rats intoxicated with doxorubicin when compared with control groups after 30 days. Rats treated with DOX + MSCs, DOX + PE and DOX + MSCs + PE observed a significant increase (p < 0.05) when compared with intoxicated groups after 30 days.
Doxorubicin induced significantly (p < 0.05) decrease serum T3 and T4 when compared to control group after 30 days. Rats treated with DOX + MSCs, DOX + PE and DOX + MSCs + PE observed a significant increase (p < 0.05) in serum T3 and T4 when compared with intoxicated groups after 30 days (Table 3).
Table 3 recorded a significant increase (p < 0.05) in serum TSH level in rats intoxicated with doxorubicin when compared with control groups after 30 days. Rats treated with DOX + MSCs, DOX + PE and DOX + MSCs + PE observed a significant decrease (p < 0.05) when compared with intoxicated groups after 30 days.
DISCUSSION
Histological examination
Doxorubicin (DOX) is one of the most effective anticancer drugs, but its clinical use is limited by life-threatening cardiotoxicity. Apart from its therapeutic cytotoxic effect on cancer cells through interacting DNA, Dox-induced ROS formation and oxidative damage. Both effects are particularly important in the pathogenesis of cardiac and hepatic injury (Stěrba et al., 2013; Yang et al., 2014).
DOX showed cardiotoxic and hepatotoxic effects in animals. It is known that antibiotics such as DOX (Kalender et al., 2002). For this reason, their prolonged use and excessive dosage cause death. These drugs disrupt basal metabolism by showing toxic effects, especially in liver and heart tissues (Kalender et al., 2005). Our biochemical, light and electron microscopic findings showed that Dox caused a hepatotoxic effect.
The present investigation showed many histopathological and ultrastructural abnormalities in the liver including inflammatory infiltration, hyperplasia, periportal fibrosis, marked disruption of hepatic cords and dilated blood sinusoids. A lot of hepatocytes showed karyomegaly and pyknotic nuclei indicating apoptosis.
The enzyme activation of doxorubicin begins with the drug conversion to a semiquinone free radical via one-electron reduction as reported previous studies, and such a reaction is catalyzed by several enzymes, including P-450 reductase (Bartoszek, 2002). In the present study, inflammatory cells forming granulomatous lesions and periportal fibrosis were detected after doxorubicin administration. Doxorubicin has been shown to induce accumulation of inflammatory cells (Saad et al., 2001), associated with increased activities of serum aminotransferases indicating hepatic damage (Deepa and Varalakshmi, 2003). Two different ways of free radical formation by DOX have been described. The first way implicates the formation of a semiquinone free radical by the action of several NADPH-dependent reductases that produce a one-electron reduction of the DOX to the corresponding DOX semiquinone. In the presence of oxygen, redox cycling of DOX-derived quinone–semiquinone yields superoxide radicals. In a second way, DOX free radicals come from a non-enzymatic mechanism that involves reactions with iron. For example, Fe3+ reacts with DOX in a redox reaction after which the iron atom accepts an electron and a Fe2+ DOX free radical complex is produced. This iron-DOX complex can reduce oxygen to hydrogen peroxide and other active oxygen species (Quiles et al., 2002). DOX generate superoxide anion radicals, H2O2 and hydroxyl radicals as a result of oxidative metabolism in rats (Doroshow, 1983). Damage at the cell level by oxidants is attenuated by antioxidant enzymes such as Superoxide dismutase (SOD), Glutathione peroxidase (GPx) and Catalase (CAT).
It has been investigated that the use of DOX results in an increased production of free radicals such as superoxide, hydroxyl radicals, and hydrogen peroxide, which have a great potential to react rapidly with lipids, which causes lipid peroxidation (Oz et al. 2005).
It is clear from the present work that the drug Doxorubicin has a toxic impact on organs especially the liver.
Doxorubicin is a very potent antitumor antibiotic. The reported acute and chronic side effects associated with doxorubicin use in clinics are the onsets of cardiotoxicity and hepatotoxicity (Kolarovic et al., 2010). In agreement with application trials on doxorubicin-induced hepatotoxicity, the present data showed that the significant increase in the activities of Aspartate Aminotransferase (AST), alkaline phosphatase (ALP) and ALT and histopathological changes in the liver were due to doxorubicin therapy. Doxorubicin toxicity is attributed to its pro-oxidant action. Many studies have described that lipid peroxidation of heart and liver cells membrane, caused by reactive oxygen species (ROS), is the main reason for tissue damage induced by doxorubicin (Dodda et al., 2014). DOX not only increases free radical production in the tissue but also decreases its ability to detoxify reactive oxygen species.
This study has shown that the abnormal histopathological changes in the liver can be attributed to increased apoptotic widely and inflammatory response.
Results of the present study indicate that the MSCs and placental extract significantly protected DOX-induced hepatotoxicity. The dose of DOX used in this study corresponds to the dose that is currently being used in the clinical practice (Chabner et al., 2001). Electron microscopy studies showed that DOX cause pathological changes in hepatocytes. This effect was seen in mitochondria, Dox-induced toxic manifestations.
Light and electron microscopic examination of DOX/MSCs group revealed an improvement of the liver structure. Most of the hepatocytes appeared nearly like those of the control group and regained their function. The damaged hepatocytes were repaired and fibrosis was resolved, resulting in an overall improvement in liver function (Oyagi et al., 2006). The liver fibrosis was also resolved after MSCs and HPE administration. The area percentage of the collagen fibers was significantly decreased as compared to DOX-alone group. MSCs ameliorated liver fibrosis by down-regulating the profibrotic genes and up-regulating anti-fibrotic hepatic genes (Ali and Masoud, 2012). Moreover, MSCs might play an inhibition role in process of Hematopoietic stem cells (HSCs) transition from the inactive state to activated state, and induce HSCs apoptosis through the release of interleukin-10 (Dai et al., 2009). Recently, it was found that BM-MSCs reduced the expression of collagen type I (Shao et al., 2014).
Upon liver injury, the body attempts to repair the damage through increasing the expression of hepatocyte growth factor (HGF), transforming growth factor-beta (TGF-β) and other cytokines to enhance hepatocyte proliferation and initiate tissue-repairing process (Paradis et al., 2001). It was found that MSCs secrete a variety of these cytokines and growth factors, which suppress the local immune system, inhibit fibrosis and apoptosis, enhance angiogenesis and stimulate mitosis and differentiation of tissue-intrinsic stem cells (Caplan et al., 2006).
Human placental extract (HPE), which is sometimes used to promote certain functions of liver or cure certain diseases such as hepatitis and liver cirrhosis through stimulating cell proliferation, has been known to promote Interleukin 8 (IL-8) expression through in vitro and liver regeneration in the CCl4-injured liver rat model (Jung et al., 2011).
Several bioactive molecules in HPE have been spotlighted in Western medicine as well as in Oriental medicine (Kang et al., 2007). Because the placenta supports fetal development through the synthesis of various molecules during pregnancy (Parolini et al., 2008), there are abundant biologically important factors, and some of these cytokines and growth factors are known to be essential for liver regeneration (Pal et al., 2002). Also, it has been reported that placenta extract stimulates tissue-repair process (Seo et al., 2006). These results are in agreement with Yamauchi et al. (2017), who showed that HPE ameliorates the pathology of MCD-induced NASH in mice by suppressing inflammation, oxidative stress, and fibrosis. Furthermore, we found that HPE directly suppresses endothelial cell damage. HPE could thus be an effective therapeutic agent with which to suppress progression from simple fatty liver to NASH.
Liver functions
One of the essential organs in the animal body is the liver, because it is primarily the site of elimination and deactivation of certain toxic xenobiotics. Transaminases (AST and ALT) play an important role in amino acids catabolism and biosynthesis. They are responsible for detoxification processes, metabolism and biosynthesis of energetic macromolecules for different essential functions (Seven et al., 2004).
The treatment of DOX led to an increase in ALT and γ-GT activity and a decrease in TP and ALB, which are marker enzymes in serum used in hepatic damage. Therefore, GPT elevated in serum. These enzymes leaked to the blood stream due to peroxidative damage of DOX to the cell membrane of the liver (Burton, 1989).
On the other hand, groups treated with MSCs and placenta extract recorded decrease in ALT and γ-GT and increase in TP and ALB: this result is in agreement with Mehrabani et al. (2019), which recorded that thioacetamide (TA) leads to increase of liver functions and MSCs ameliorate effect of TA-induced model of rat fibrosis, and the treatment of liver fibrosis with BMSCs leads to a significant reduction in the number of inflammatory cells and collagen deposition in the hepatic parenchyma. Liver function tests denoted a decrease in serum: this indicated the healing effect of MSCs, as the secretion of many bioactive factors by MSCs can provide a microenvironment for the rearrangement of liver injuries (Togel et al., 2007). These factors can inhibit scarring (i.e., fibrosis) and apoptosis, promote angiogenesis and stimulate host progenitor cells for division and differentiation into functional regenerative units (Mehrabani et al., 2013). Furthermore, the trophic effects of MSCs can have prominent clinical use (Mehrabani et al., 2016).
In transplanted encapsulated human MSCs and in the mouse model of liver fibrosis, it was observed that MSC-derived soluble molecules were responsible for antifibrotic effects (Meier et al., 2015). The effect of BMSCs on hepatic fibrosis was evaluated in a TA-induced cirrhotic rat model, and the results showed that the treatment with BMSCs could attenuate hepatic fibrosis (Jang et al., 2014).
Several bioactive molecules in placenta extract have been spotlighted in Western medicine as well as in Oriental medicine (Yeom et al., 2003; Kang et al., 2007). Because the placenta supports fetal development through the synthesis of various molecules during pregnancy (Parolini et al., 2008), there are abundant biologically important factors, and some of these cytokines and growth factors are known to be essential for liver regeneration (Pal et al., 2002). Also, it has been reported that placenta extract stimulates tissue repair process (Seo et al., 2006), has therapeutic effects in chronic non-healing wounds (Shukla et al., 2004; Tiwary et al., 2006), and has anti-inflammatory effects (Sur et al., 2003). However, despite the identification of biologically active molecules and trials for several diseases, precise underlying mechanisms remain largely unknown and warrant further investigation (Uehara et al., 1995).
In agreement with Jung et al. (2011), placental extract administration could improve liver function after treated rat with CCl4 and led to an increase in liver enzymes, since the liver is the main organ responsible for the biotransformation and subsequent detoxification of xenobiotics, the enzymes for biotransformation are critical. Therefore, we investigated the alteration of enzymes after transplantation of MSCs into the mouse liver injured by CCl4 administration, and found a reduction in AST after injection with CCl4, which causes increase it because of the role of MSCs (Cho et al., 2012) and placenta extract which lows from free radicals.
The present study did record a significant increase in cholesterol, triglyceride and LDL-C concentration and decrease in HDL-C concentration in rats exposed to DOX when compared to control groups, due to the fact that cholesterol is crucial for maintaining cellular homeostasis. It is a precursor for steroid hormones and a component of membrane bilayers that is essential for their integrity and to enable cell proliferation (Simons and Ikonen, 1997). In addition, cholesterol depletion from the cell membrane results in lipid raft internalization from the cell membrane, causing the deregulation of cellular signaling that leads to cell death (Li et al., 2006; Yun et al., 2019). Some cholesterol is provided by the diet, but it is primarily synthesized in the liver and distributed to cells via the bloodstream (Kuzu et al., 2016). On the other hands, stem cell and placenta extract induce ameliorate effect on lipid profile.
The groups treated with DOX recorded a decrease in T3, T4 and increase in TSH: these results in agreement with Olson et al., (2005), due to the fact that T3 and T4 are important regulators of metabolism and physiology of most normal tissues. Cytochrome P450 family 3A members are drug-metabolizing enzymes involved in the activation and detoxification of several drugs (Flaqué et al., 2019), which happen in the liver, as the liver is responsible for conversion about 80 percent of T4 to T3 inside it.
Placenta extract and MSCs recorded ameliorative effect against DOX.
Conclusion
It could be concluded that, as indicated by biochemical and histological Changes, DOX has deleterious effects on the liver. By increasing liver functions and Apoptosis, MSC and Placental extract have protective effects against DOX-induced hepatotoxicity. Accordingly, prohibiting the use of DOX and using MSCs and Placental extract as hepatoprotective agents are highly recommended.
ACKNOWLEDGEMENTS
We deeply acknowledge Dr. Ahmed Belal Mehany, lecturer of genetic engineering, for her assistance in performing the biochemical investigations. This work was not supported by any funds from any organization.
Related articles
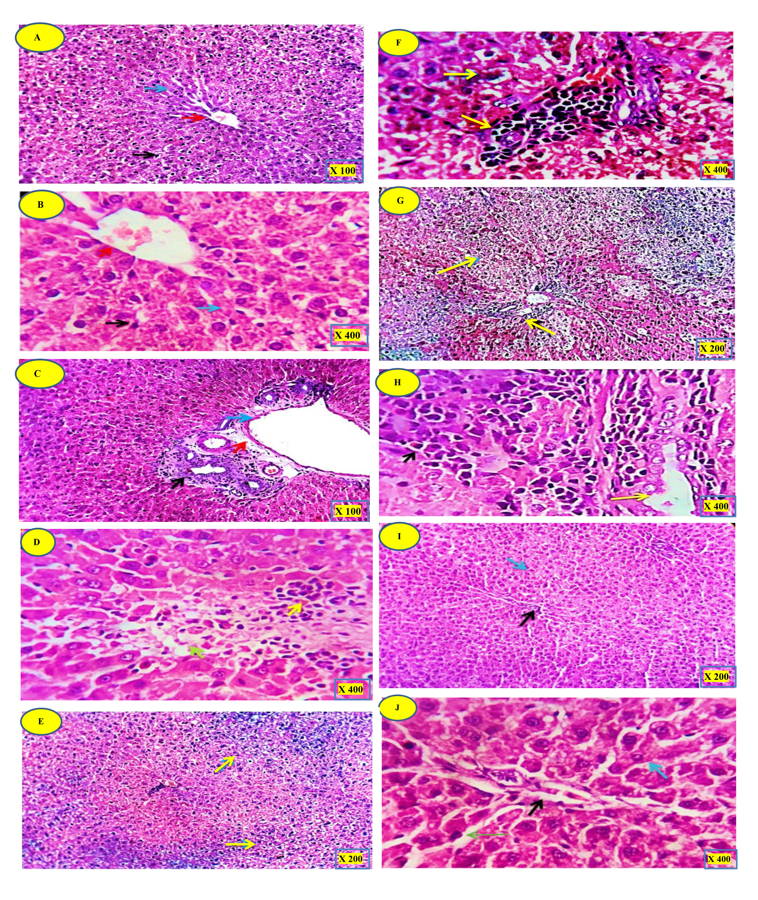 Fig. 1.- (A, B): Liver control rat demonstrating normal hepatocyte structure. (C, D): DOX group. (E, F): DOX + MSCs group. (G, H): DOX + PE group. (I, J): DOX + MSCs + PE. H&E staining. Magnifications: A, C = x 100; B, D, F, H, J = x 400; E, G, I = x 200. For explanation see results section.
Fig. 1.- (A, B): Liver control rat demonstrating normal hepatocyte structure. (C, D): DOX group. (E, F): DOX + MSCs group. (G, H): DOX + PE group. (I, J): DOX + MSCs + PE. H&E staining. Magnifications: A, C = x 100; B, D, F, H, J = x 400; E, G, I = x 200. For explanation see results section.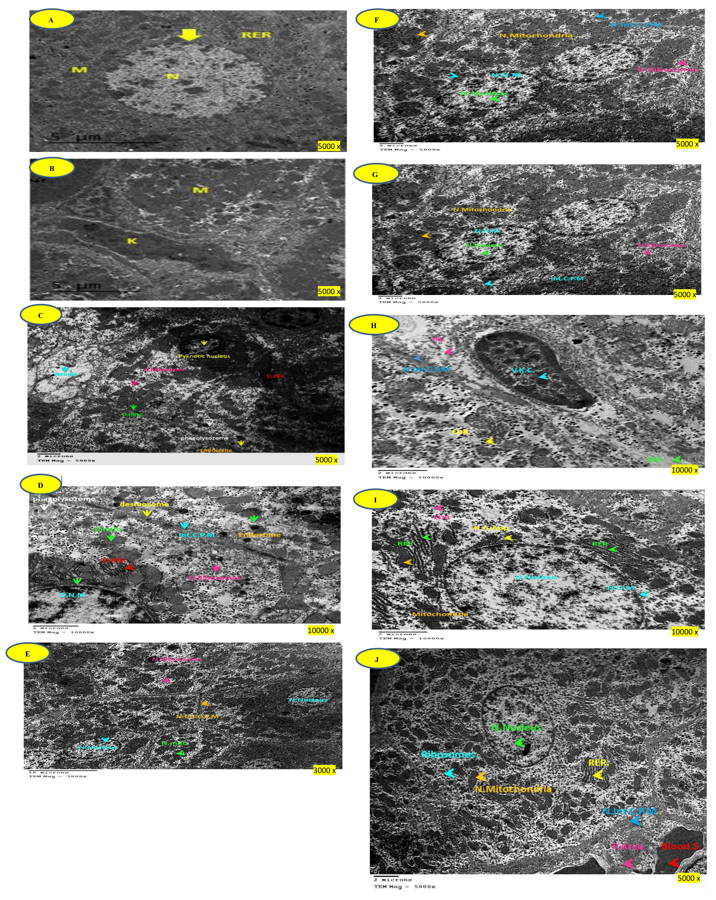 Fig. 2.- (A, B): Electron micrograph of liver control rat demonstrating normal hepatocyte structure. Scale bar = 5 microns, TEM magnification: x 5000. (C, D): DOX group. Scale bars = 2 microns. C = TEM magnification 5000x, D =TEM magnification 10000x. (E, F): DOX + MSCs group. Scale bars = 10 microns. E = TEM magnification 3000x, F = TEM magnification 5000x. (G, H): DOX + PE group. Scale bars = 2 microns. G = TEM magnification 5000x, H = TEM magnification 10000x. (I, J): DOX + MSCs + PE group. Scale bars = 2 microns. I = TEM magnification 10000x, J = TEM magnification 5000x. For explanation see results section.
Fig. 2.- (A, B): Electron micrograph of liver control rat demonstrating normal hepatocyte structure. Scale bar = 5 microns, TEM magnification: x 5000. (C, D): DOX group. Scale bars = 2 microns. C = TEM magnification 5000x, D =TEM magnification 10000x. (E, F): DOX + MSCs group. Scale bars = 10 microns. E = TEM magnification 3000x, F = TEM magnification 5000x. (G, H): DOX + PE group. Scale bars = 2 microns. G = TEM magnification 5000x, H = TEM magnification 10000x. (I, J): DOX + MSCs + PE group. Scale bars = 2 microns. I = TEM magnification 10000x, J = TEM magnification 5000x. For explanation see results section.ALI G, MASOUD MS (2012) Bone marrow cells ameliorate liver fibrosis and express albumin after transplantation in CCl4-induced fibrotic liver. Saudi J Gastroenterol, 18(4): 263-267.
ALLAIN C, POON L, CHAN C, RICHMOND W, FU P (1974) Enzymatic determination of total serum cholesterol. Clin Chem, 20(4): 470-475.
AYDIN B, UNSAL M, SEKEROGLU ZA, GÜLBAHAR Y (2011) The antioxidant and antigenotoxic effects of pycnogenol® on rats treated with cisplatin. Biol Trace Elem Res, 142(3): 638-650.
BARTOSZEK A (2002) Metabolic activation of adriamycin by NADPH-cytochrome P450 reductase; overview of its biological and biochemical effects. Acta Biochim Pol, 49(2): 323-31.
BECK JR (1986) Laboratory decision science applied to chemometrics: strategic testing of thyroid function. Clin Chem, 32(9): 1707-1713.
BERGMEYER HU, HORDER M, REY J (1986) Approved recommendation on IFCC methods for the measurement of catalytic enzymes. Part 2: IFCC method for aspartate aminotransferase. J Clin Chem Clin Biochem, 24: 497-510.
BURSTEIN M, SCHOLNICK HR, MORFIN R (1970) Rapid method for the isolation of lipoproteins from human serum by precipitation with polyanions. J Lipid Res, 11(6): 583-595.
BURTON GW (1989) Antioxidant action of carotenoids. J Nutr, 119: 109‑111.
CALDWELL G, GOW SM, SWEETING VW, KELLETT HA, BECKETT GJ, SETH J, TOFT AD (1985) New strategy for thyroid function testing. Lancet, 325(8438): 1117-1119.
CAPLAN AI, DENNIS JE (2006) Mesenchymal stem cells as trophic mediators. J Cell Biochem, 98(5): 1076-1084.
CHABNER BA, RYAN DP, PAZ-ARES L, GARCIACARBONEVO R, CALABRESI P (2001) Antineoplastic agents. In: Hardman JG, Limbird LE, Gilman AG (eds.) Goodman and Gilman’s the Parmacological Basis of Therapeutics. McGraw Hill Companies Inc., USA, pp 1389-1459.
CHANG YL, LEE HJ, LIU ST, LIN YS, CHEN TC, HSIEH TY, HUANG HS, HUANG SM (2011) Different roles of p53 in the regulation of DNA damage caused by 1,2 heteroannelatedanthraquinones and doxorubicin. Int J Biochem Cell Biol, 43(12): 1720-1728.
CHO KA, WOO SY, SEOH JY, HAN HS, RYU KH (2012) Mesenchymal stem cells restore CCl4‐induced liver injury by an antioxidative process. Cell Biol Int, 36(12): 1267-1274.
DAI LJ, LI HY, GUAN LX, RITCHIE G, ZHOU JX (2009) The therapeutic potential of bone marrow-derived mesenchymal stem cells on hepatic cirrhosis. Stem Cell Res, 2(1): 16-25.
DEEPA PR, VARALAKSHMI P (2003) Protective effect of low molecular weight heparin on oxidative injury and cellular abnormalities in adriamycin-induced cardiac and hepatic toxicity. Chemico-Biological Interactions, 146(2): 201-210.
DODDA D, CHHAJED R, MISHRA J (2014) Protective effect of quercetin against acetic acid induced inflammatory bowel disease (IBD) like symptoms in rats: Possible morphological and biochemical alterations. Pharmacol Reports, 66(1): 169-173.
DOUMAS BT, WATSON WA, BIGGS HG (1971) Albumin standards and the measurement of serum albmin with bromocresol green. Clin Chem Acta, 31(1): 87-96.
EL-SAYYAD HI, ISMAIL MF, SHALABY FM, ABOU-EL-MAGD RF, GAUR RL, FERNANDO A, OUHTIT A (2009) Histopathological effects of cisplatin, doxorubicin and 5-flurouracil (5-FU) on the liver of male albino rats. Int J Biol Sci, 5(5): 466.
EL-SHEIKH AA, MORSY MA, MAHMOUD MM, RIFAAI RA, ABDELRAHMAN AM (2010) Effect of coenzyme-q10 on Doxorubicin-induced nephrotoxicity in rats. Adv Pharmacol Sci, 2012.
FLAQUÉ MCD, CAYROL MF, STERLE HA, DEL ROSARIO ASCHERO M, ALBUJA JAD, ISSE B, FARÍAS RN, CERCHIETTI L, ROSEMBLIT C, CREMASCHI GA (2019) Thyroid hormones induce doxorubicin chemosensitivity through enzymes involved in chemotherapy metabolism in lymphoma T cells. Oncotarget, 10(32): 3051-3065.
FOSSATI P, PRENCIPE L (1982) Serum triglycerides determined colorimetrically with an enzyme that produces hydrogen peroxide. Clin Chem Acta, 28(10): 2077-2080.
GIANNI L, SALVATORELLI E, MINOTTI G (2007) Anthracycline cardiotoxicity in breast cancer patients: Synergism with trastuzumab and taxanes. Cardiovasc Toxicol, 7(2): 67-71.
GORNAL AC, BARDWILL CJ, DAVID MM (1949) Determination of serum proteins by means of the biuret reaction. J Biol Chem, 177(2): 751-766.
HARMS PG, OJEDA SR (1974) A rapid and simple procedure for chronic cannulation of the rat jugular vein. J Appl Physiol, 36(3): 391-392.
HONG JW, LEE WJ, HAHN SB, KIM BJ, LEW DH (2010) The effect of human placenta extract in a wound healing model. Ann Plastic Surg, 65(1): 96-100.
HU X, YU SP, FRASER JL, LU Z, OGLE ME, WANG JA, WEI L (2008) Transplantation of hypoxia-preconditioned mesenchymal stem cells improves infarcted heart function via enhanced survival of implanted cells and angiogenesis. J Thorac Cardiovasc Surg, 135(4): 799-808.
JOHANNESSEN JV (1978) Electron microscopy in human medicine. McGraw-Hill International Book Co., New York.
JOYCE NC, HARRIS DL, MARKOV V, ZHANG Z, SAITTA B (2012) Potential of human umbilical cord blood mesenchymal stem cells to heal damaged corneal endothelium. Mol Vis, 18: 547-564.
JUNG J, LEE HJ, LEE JM, NA KH, HWANG SG, KIM GJ (2011) Placenta extract promote liver regeneration in CCl4-injured liver rat model. Int Immunopharmacol, 11(8): 976-984.
KALENDER S, KALENDER Y, ATES A, YEL M, OLCAY E, CANDAN S (2002) Protective role of antioxidant Vitamin E and catechin on idarubicin-induced cardiotoxicity in rats. Braz J Med Biol Res, 35(11): 1379-1387.
KALENDER Y, YEL M, KALENDER S (2005) Doxorubicin hepatotoxicity and hepatic free radical metabolism in rats. The effects of vitamin E and catechin. Toxicology, 209(1): 39-45.
KANG SS, WOO SS, IM J, YANG JS, YUN CH, JU HR (2007) Human placenta promotes IL-8 expression through activation of JNK/SAPK and transcription factors NF-kappaB and AP-1 in PMA-differentiated THP-1 cells. Int Immunopharmacol, 7(11): 1488-1495.
KOLAROVIC J, POPOVIC M, ZLINSKÁ J, TRIVIC S, VOJNOVIC M (2010) Antioxidant activities of celery and parsley juices in rats treated with doxorubicin. Molecules, 15(9): 6193-6204.
KON E, FILARDO G, ROFFI A, DI MARTINO A, HAMDAN M, DE PASQUAL L (2010) Bone regeneration with mesenchymal stem cells. Clin Cases Miner Bone Metab, 9(1): 24-27.
KONG MH, LEE EJ, LEE SY, CHO SJ, HONG YS, PARK SB (2008) Effect of human placental extract on menopausal symptoms, fatigue, and risk factors for cardiovascular disease in middle-aged Korean women. Menopause, 15(2): 296-303.
KUZU OF, NOORY MA, ROBERTSON GP (2016) The role of cholesterol in cancer. Cancer Res, 76(8): 2063-2070.
KWON TR, OH CT, PARK HM, HAN HJ, JI HJ, KIM BJ (2015) Potential synergistic effects of human placental extract and minoxidil on hair growth‐promoting activity in C 57 BL/6 J mice. Clin Exp Dermatol, 40(6): 672-681.
LAHOTI TS, PATEL D, THEKKEMADOM V, BECKETT R, RAY SD (2012) Doxorubicin-induced in vivo nephrotoxicity involves oxidative stress-mediated multiple pro-and anti-apoptotic signaling pathways. Curr Neurovasc Res, 9(4): 282-295.
LI YC, PARK MJ, YE SK, KIM CW, KIM YN (2006) Elevated levels of cholesterol-rich lipid rafts in cancer cells are correlated with apoptosis sensitivity induced by cholesterol-depleting agents. Am J Pathol, 168(4): 1107-1118.
MA Y, YANG L, MA J, LU L, WANG X., REN J, YANG J (2017) Rutin attenuates doxorubicin-induced cardiotoxicity via regulating autophagy and apoptosis. Biochim Biophys Acta (BBA)-Molecular Basis of Disease, 1863(8): 1904-1911.
MEHRABANI D, MEHRABANI G, ZARE S, MANAFI A (2013) Adipose-derived stem cells (ADSC) and aesthetic surgery: a mini review. World J Plast Surg, 2(2): 65-70.
MEHRABANI D, MOJTAHEDJABERI F, ZAKERINIA M, HADIANFARD MJ, JALLI R, TANIDEH N, ZARE S (2016) The healing effect of bone marrow-derived stem cells in knee osteoarthritis: a case report. World J Plast Surg, 5(2): 168-174.
MEHRABANI D, KHAJEHAHMADI Z, TAJIK P, TAMADON A, RAHMANIFAR F, ASHRAF M, TANIDEH N, ZARE S (2019) Regenerative effect of bone marrow-derived mesenchymal stem cells in thioacetamide-induced liver fibrosis of rats. Arch Razi Inst, 74(3): 279-286.
MOHAN M, KAMBLE S, SATYANARAYANA J, NAGESHWAR M, REDDY N (2011) Protective effect of solanum torvum on doxorubicin-induced hepatotoxicity in rats. Int J Drug Dev Res, 3(3): 131-138.
OLSON RD, GAMBLIEL HA, VESTAL RE, SHADLE SE, CHARLIER HA, CUSACK BJ (2005) Doxorubicin cardiac dysfunction. Cardiovasc Toxicol, 5(3): 269-283.
OYAGI S, HIROSE M, KOJIMA M, OKUYAMA M, KAWASE M, NAKAMURA T, OHGUSHI H, YAGI K (2006) Therapeutic effect of transplanting HGF-treated bone marrow mesenchymal cells into CCl4-injured rats. J Hepatol, 44(4): 742-748.
ÖZ E, ILHANMN (2006) Effects of melatonin in reducing the toxic effects of doxorubicin. Mol Cell Biochem, 286(1-2): 11-15.
PAGET GE, BARNES JM (1964) Toxicity tests in evaluation of drug activities pharmacometries (Laurence DR, Bacharach AL, eds.). Academic Press, London, New York.
PAL P, MALLICK S, MANDAL SK, DAS M, DUTTA AK, DATTA PK (2002) A human placental extract: in vivo and in vitro assessments of its melanocyte growth and pigment inducing activities. Int J Dermatol, 41(11): 760-767.
PARADIS V, YOUSSEF N, DARGÈRE D, BÂ N, BONVOUST F, DESCHATRETTE J, BEDOSSA P (2001) Replicative senescence in normal liver, chronic hepatitis C, and hepatocellular carcinomas. Hum Pathol, 32(3): 327-332.
PAROLINI O, ALVIANO F, BAGNARA GP, BILIC G, BUHRING HJ, EVANGELISTA M (2008) Concise review: isolation and characterization of cells from human term placenta: outcome of the first international Workshop on Placenta Derived Stem Cells. Stem Cells, 26(2): 300-311.
QUERTAINMONT R, CANTINIEAUX D, BOTMAN O, SID S, SCHOENEN J, FRANZEN R (2012) Mesenchymal stem cell graft improves recovery after spinal cord injury in adult rats through neurotrophic and pro-angiogenic actions. PLoS One, 7(6): e39500.
RASH AR, ABDELLA EM (2010) Modulatory effects of Rosemary leaves Aqueous on doxorubicin-induced histological lesions, apoptosis and oxidative stress in mice. Iranian J Cancer Prev, 3: 1-22.
ROSALKI SB, TARLOW D, RAU D (1971) Plasma gamma-glutamyl transpeptidase elevation in patients receiving’ enzyme-inducing drugs. Lancet, 298(7720): 376-377.
ROZANOVA SL, NAUMENKO EI, ROZANOVA ED, NARDID OA (2010) Change of anioxidative properties of human placental extracts after freezing. Problems Cryobiol, 20(3): 288-297.
SAAD SY, NAJJA TA, AL-RIKABI AC (2001) The preventive role of deferoxamine against acute doxorubicin-induced cardiac, renal and hepatic toxicity in rats. Pharmacol Res, 43(3): 211-218.
SALEM HK, THIEMERMANN C (2010) Mesenchymal stromal cells: current understanding and clinical status. Stem Cells, 28(3): 585-596.
SEO TB, HAN IS, YOON JH, SEOL IC, KIM YS, JO HK (2006) Growth-promoting activity of Hominis Placenta extract on regenerating sciatic nerve. Acta Pharmacol Sin, 27(1): 50-58.
SEVEN A, GUZEL S, SEYMEN O, CIVELEK S, BOLAYIRLI M, UNCU M, BURCAK G (2004) Effects of vitamin E supplementation on oxidative stress in streptozotocin induced diabetic rats: investigation of liver and plasma. Yonsei Med J, 45(4): 703-710.
SHAO CH, CHEN SL, DONG TF, CHAI H, YU Y, DENG L, WANG Y, CHENG F (2014) Transplantation of bone marrow-derived mesenchymal stem cells after regional hepatic irradiation ameliorates thioacetamide-induced liver fibrosis in rats. J Surg Res, 186(1): 408-416.
SHUKLA VK, RASHEED MA, KUMAR M, GUPTA SK, PANDEY SS (2004) A trial to determine the role of placental extract in the treatment of chronic non-healing wounds. J Wound Care, 13(5): 177-179.
SIMONS K, IKONEN E (1997) Functional rafts in cell membranes. Nature, 387(6633): 569-572.
SNYKERS S, VANHAECKE T, PAPELEU P, LUTTUN A, JIANG Y, VAN DER HEYDEN Y, VERFAILLIE C, ROGIERS V (2006) Sequential exposure to cytokines reflecting embryogenesis: the key for in vitro differentiation of adult bone marrow stem cells into functional hepatocyte-like cells. Toxicol Sci, 94(2): 330-341.
SONNLEITNER D, HUEMER P, SULLIVAN DY (2000) A simplified technique for producing platelet-rich plasma and platelet concentrate for intraoral bone grafting techniques: a technical note. Int J Oral Maxillofac Implants, 15(6): 879-882.
STĚRBA M, POPELOVÁ O, VÁVROVÁ A, JIRKOVSKÝ E, KOVAŘÍKOVÁ P, GERŠL V (2013) Oxidative stress, redox signaling, and metal chelation in anthracycline cardiotoxicity and pharmacological cardioprotection. Antioxid Redox Signal, 18(8): 899-929.
SUR TK, BISWAS TK, ALI L, MUKHERJEE B (2003) Anti-inflammatory and anti-platelet aggregation activity of human placental extract. Acta Pharmacol Sin, 24(2): 187-192.
SUVARNA KS, LAYTON C, BANCROFT JD (2013) Bancroft’s Theory and Practice of Histological Techniques, E-Book. Elsevier Health Sciences, 7th edition.
THIPPESWAMY AH, SHIRODKAR A, KOTI BC, SADIQ AJ, PRAVEEN DM, SWAMY AH, PATIL M (2011) Protective role of Phyllantusniruri extract in doxorubicin-induced myocardial toxicity in rats. Indian J Pharmacol, 43(1): 31-35.
TIWARY SK, SHUKLA D, TRIPATHI AK, AGRAWAL S, SINGH MK, SHUKLA VK (2006) Effect of placental-extract gel and cream on non-healing wounds. J Wound Care, 15(7): 325-328.
TOGEL F, WEISS K, YANG Y, HU Z, ZHANG P, WESTENFELDER C (2007) Vasculotropic, paracrine actions of infused mesenchymal stem cells are important to the recovery from acute kidney injury. Am J Physiol Renal Physiol, 292(5): F1626-F1635.
UEHARA Y, MINOWA O, MORI C, SHIOTA K, KUNO J, NODA T, KITAMURA N (1995) Placental defect and embryonic lethality in mice lacking hepatocyte growth factor/scatter factor. Nature, 373(6516): 702-705.
WHEELER MH, LAZARUS JH (1994) Diseases of the Thyroid. Chapman and Hall Medical, London, Glasgow, Weinheim, New York, Tokyo, Melbourne, Madras, pp 107-115.
WIELAND H, SEIDEL D (1982) Improved assessment of plasma lipoprotein patterns. IV. Simple preparation of a lyophilized control serum containing intact human plasma lipoproteins. Clin Chem, 28(6): 1335-1337.
WU J, WANG C, LIU Q, YANG T, ZHANG Q, PENG J, LIU K (2008) Protective effect of JBP485 on concanavalin A-induced hepatocyte toxicity in primary cultured rat hepatocytes. Eur J Pharmacol, 589(1-3): 299-305.
YAMAUCHI A, KAMIYOSHI A, KOYAMA T, IINUMA N, YAMAGUCHI S, MIYAZAKI H, HIRANO E, KAKU T, SHINDO T (2017) Placental extract ameliorates non-alcoholic steatohepatitis (NASH) by exerting protective effects on endothelial cells. Heliyon, 3(9): e00416.
YAMAUCHI A, KAMIYOSHI A, SAKURAI T, MIYAZAKI H, HIRANO E, LIM HS, KAKU T, SHINDO T (2019) Placental extract suppresses cardiac hypertrophy and fibrosis in an angiotensin II-induced cachexia model in mice. Heliyon, 5(10): e02655.
YANG F, TEVES SS, KEMP CJ, HENIKOFF S (2014) Doxorubicin, DNA torsion, and chromatin dynamics. Biochim Biophys Acta, 1845(1): 84-89.
YEOM MJ, LEE HC, KIM GH, SHIM I, LEE HJ, HAHM DH (2003) Therapeutic effects of Hominis placenta injection into an acupuncture point on the inflammatory responses in subchondral bone region of adjuvant-induced polyarthritic rat. Biol Pharm Bull, 26(10): 1472-1477.
YUN UJ, LEE JH, SHIM J, YOON K, GOH SH, YI EH, YE AK, LEE JS, LEE H, PARK J, LEE IH (2019) Anti-cancer effect of doxorubicin is mediated by downregulation of HMG-Co A reductase via inhibition of EGFR/Src pathway. Lab Invest, 99(8): 1157-1172.
ZAAHKOUK SMA, BAKRY S, MANSOUR A, IBRAHIM RH (2015) Therapeutic role of mesenchymal stem cells in cisplatin induced renal failure in adult male rats. Adv Biol Res, 9(3): 201-209.
ZENG X, YU SP, TAYLOR T, OGLE M WEI L (2012) Protective effect of apelin on cultured rat bone marrow mesenchymal stem cells against apoptosis. Stem Cell Res, 8(3): 357-367.