INTRODUCTION
Diabetes mellitus (DM) was once considered to be a disease of trivial significance but has now become a major public health challenge of the 21st century, especially in developing countries (Venkataramana et al., 2013). DM is defined as a clinical syndrome which is characterized by hyperglycemia due to absolute or relative deficiency of insulin, as well as disturbances of carbohydrate, fat and protein metabolism associated with absolute or relative deficiency in insulin secretion or insulin action (Jayakar and Suresh, 2003; World Health Organization 2013). DM can be divided into two principal forms: Type I diabetes mellitus (Type I DM) and Type II diabetes mellitus (Type II DM). Type I DM is characterized by loss of insulin producing beta cells of the islets of Langerhans in the pancreas leading to insulin deficiency. This type can be further classified as immune-mediated or idiopathic. The popular cause of Type I DM is of the immune-mediated nature, in which beta cell loss is a T-cell-mediated autoimmune attack (Rother, 2007). Type II DM is characterized by insulin resistance and/or abnormal insulin secretion. Individuals with Type II DM are not dependent on exogenous insulin, but may require it for control of blood glucose levels if this is not achieved with diet alone or with oral hypoglycemic agents (Venkataramana et al., 2013).
The past two decades has heralded an upsurge in the number of people diagnosed with DM worldwide (Ukwani and Igbokwu, 2015). It is one of the major causes of premature death worldwide. Every ten seconds, a person dies from diabetes-related causes, mainly from cardiovascular complications. In 2010, it was reported that about 6.6% people (representing 285 million people) suffer from diabetes (International Diabetes Federation, 2017, Cho et al., 2018). It is predicted that about 366 million people are likely to be diabetic by the year 2030 (Wild et al., 2004). In sub-Saharan Africa, Nigeria has the highest number of people with DM with an estimated 3.9 million people (World Health Assembly, 2013). In addition, there are about 1.8 million undiagnosed Nigerians suffering from DM (Dahiru et al., 2016). Treatment of DM has always included the administration of insulin and oral hypoglycemic agents in conjunction with dietary counselling and life style modification (Bella, 1990). Insulin therapy and oral hypoglycemic agents offer effective glycemic control; yet, their shortcomings limit their usage (Anuradha et al., 2004). The disadvantages of oral and injected hypoglycemic agents include: injection-site pain or abscess, cost implications, decreased appetite, weight gain, risk of hypoglycemia and gastro-intestinal discomfort (Valeron and de Pablos-Velasco, 2013). Leptadenia hastata (Pers) Decne (Family-Asclepiadaceae), commonly known as yadiya, is an edible non-domesticated vegetable collected in wild throughout Africa. It is a voluble herb with creeping latex stems, glabescent leaves, glomerulus and racemus flowers, as well as follicle fruits. Wild plants like Leptadenia hastata provide food security during seasonal changes and are used medicinally in many areas. The breeders commonly used the leaf and stems for their parasitic activity and against placental retention (Bello et al., 2011).
Ethno-botanical information obtained from traditional medical practitioners in northern Nigeria and during the course of this study revealed that it is used locally for the treatment of diabetes mellitus. Its antimicrobial effect has been also reported by Aliero and Wara (2009). DM induced by streptozotocin and alloxan in laboratory animals have been reported in causing pathological alterations in the liver, which vary from steatosis to steatohepatitis and liver fibrosis (Bilal et al., 2016). Currently available therapies for diabetes have a number of adverse effects, and as such there is an increased interest in the search for more effective and safer hypoglyceamic agents to be administered in lieu of the existing medication. Leptadenia hastata is traditionally used in the management of DM and its hypoglycemic activities has been previously reported. However, there is the dearth of scientific evidence on the effects of the plant on organs like the liver, which serves as the body’s primary organ for detoxification and metabolism. It is also a site for biotransformation by which a toxic compound gets transformed to less harmful form to reduce toxicity. However, toxic compounds could damage the liver cells and produce hepatotoxicity (Rajeshkumar, 2010). In view of the above, the present study was aimed to evaluate the effects of Leptadenia hastata on the histology of the liver in streptozotocin-induced diabetic rats.
MATERIALS AND METHODS
Collection, identification and extraction of plant material
Leptadenia hastata was collected from a garden, the leaves were harvested, washed and shade-dried for a period of two weeks, and then ground to powder using a mortar and pestle. The powder was sieved to obtain the fine powder; it was then labeled and stored for use.
Maceration technique as described by Azwanida (2015) was used for extraction in the current study. The leaf powder weighing 500g was dissolved in 3 liters of n-hexane in a 5-liter stoppered container. Maceration involved soaking the plant, which is allowed to stand at room temperature for a period of 3 days at the minimum with periodic agitation. The process softened and broke the plant’s cell wall to release the soluble phytochemicals. After 3 days, the mixture was filtered using Whatman’s filter paper. The resulting n-hexane filtrate was concentrated to dryness in-vacuo using an evaporator and the resulting powder was kept in an air–tight container and refrigerated.
Experimental animals
All experiments were performed using Wistar albino rats of both sexes. A total of 30 albino rats weighing 135-190 g were used. The rats were obtained from the National Veterinary Research Institute (NVRI) Vom, Plateau State, Nigeria. They were kept in the Animal house of the Department of Human Anatomy, Faculty of Basic Medical Sciences, College of Medical Sciences, University of Maiduguri, Borno State for two weeks prior to the start of the experiment to acclimate to the new environment. The rats were weighed and maintained under controlled conditions of humidity (50-60%), temperature of 22°C±3°C, 12 hours light and 12 hours dark as well as adequate ventilation. They were fed with pelletized ECWA (Jos) feed and water ad libitum.
Experimental design
Diabetes was induced in twenty rats. These diabetic rats were divided into four groups (3-6) of 5 rats each. Rats in groups 3-6 received olive oil, 100 mg/kg of extract, 200 mg/kg of extract and insulin (6 IU/kg), respectively. In addition, 10 non-diabetic rats were grouped into 1 and 2. They received olive oil and 200 mg/kg of extract, respectively for 28 days. Olive oil was used as vehicle to dissolve the extract as it was not soluble in water.
Experimental induction of diabetes in rats
Hyperglycemia was induced in overnight fasted Wistar rats by a single intra-peritoneal injection of 50 mg/kg streptozotocin (Bristol-Sigma, Bristol Scientific Company, Missouri, United States of America) dissolved in 0.1M ice-cold sodium citrate buffer, (pH = 4.5), immediately before use in a volume of 1 ml/kg body weight as described by Etuk (2010). Hyperglycemia was confirmed by the elevated plasma glucose levels determined in tail blood sample using a glucometer (Roche, Germany). Rats whose fasting blood glucose levels exceeded 250 mg/dl (13 mmol/dl) after one week were considered as diabetic and used for the study. Urinalysis was also carried out to confirm diabetes in all groups according to a method adopted by Houcine et al. (2011).
Administration of the extract
The extract was given orally for a period of 28 days and administration was carried according to OECD guidelines (OECD TG 407) [European Chemicals Agency, 2016]. At the end of the experimental period, the animals were sacrificed by inducing sleep by injecting with ketamine hydrochloride (Rotexmedica, Trittau, Germany); the liver was removed and fixed in 10% formalin in preparation for routine histological processing.
Use of glucometer strips to determine blood glucose level
Serum glucose levels were determined weekly by testing the blood obtained from the tail of rats in all groups using Acucheck glucometer strips. The tip of the rat tail was swabbed with a disinfectant and then the tip of the tail pierced using an Accu-check Softclix lancet, and then the blood was applied to an Accu-check strip and then the strip, which was inserted into the Accu-check blood glucose meter to obtain a reading. The blood glucose level was read off the meter and the result recorded.
Analysis of serum glucose level
At the end of 28 days, the serum glucose levels were determined using Labkit protocol (Barcelona, Spain) kits using the glucose oxidase method as described by Kanagasabapathy and Kumari (2000). The mass spectrometer was adjusted to zero with blank or reagent. The sample was pipetted into a curvette along with the following solutions: in the first test tube, 1.0 ml of the reagent was added, in the second test tube, 1.0 ml of reagent and 10 μL of calibrator was added and in the third test tube, 1.0 ml of reagent and 10 μL was added. These solutions were mixed and incubated for 5 minutes at 37oC or 10 minutes at room temperature (15-25oC). The absorbance of the samples and calibrator were observed against the blank and calculated if the color was observed to be stable for at least 30 minutes.
Calculations:
Glucose (mg/dl) = (A) Sample x 100 (Calibrator conc)
(A) Calibrat
or Calibrator Conversion factor: mg/dL x 0.555 = mmol/L
Analysis of the histological sections
The images of the histological sections were obtained using an Amscope light microscope (MBJX-ISCOPE, Los Angeles) fitted with a digital camera (M500, X 64, version 3.7) under several magnifications. Images of the histological sections were obtained using 10X objective lens and were also analyzed using Amscope Image software. An ocular micrometer, which was previously standardized with a stage micrometer, was also used to measure areas of interest in the histological slides. The results were analyzed using GraphPad Instat Software (Version 3.75).
The histological section of the liver in all groups were morphometrically analyzed to observe the following measurements.
a) Diameter of the central vein (μm)
b) Width of the sinusoids (μm)
c) Width of the hepatocytes (μm)
RESULTS
Histological observations in the liver
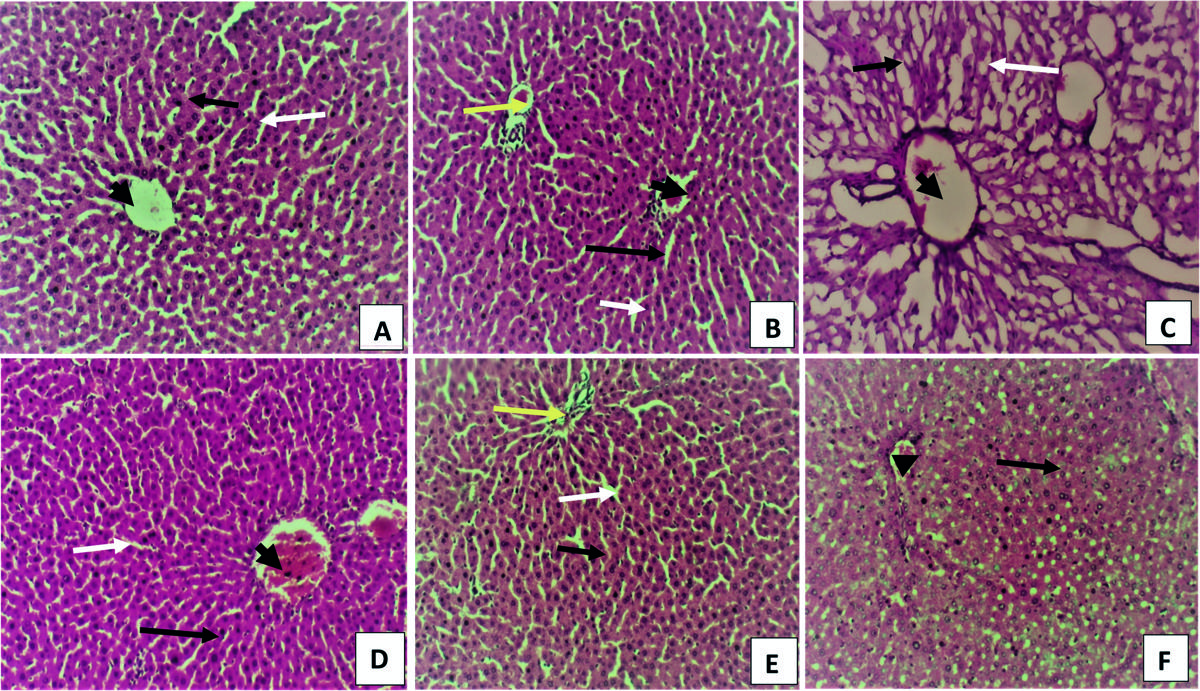
The micrographs of the liver in all groups are represented by Figs. 1A-F and 2A-F. Liver sections obtained from normal group (group 1) revealed normal histological architecture with regular hepatic lobules with central veins, and peripheral portal areas were observed (Fig. 1A). Hepatocytes with sinusoidal spaces were extending radially from the central veins to the boundaries of portal areas. There was mild congestion of the hepatic vein in the rats in Group II. The rats in Group III showed wider sinusoids (white arrow) and degenerative changes to the hepatocytes (white arrow). The central veins (black arrow head) in this group were wider and more dilated than in the other groups. In Group IV, V and VI, the architecture of the liver was normal (Figs. 1D, E and F), with clear sinusoid (white arrow), hepatic cords (black arrow) and central vein (black arrow head).
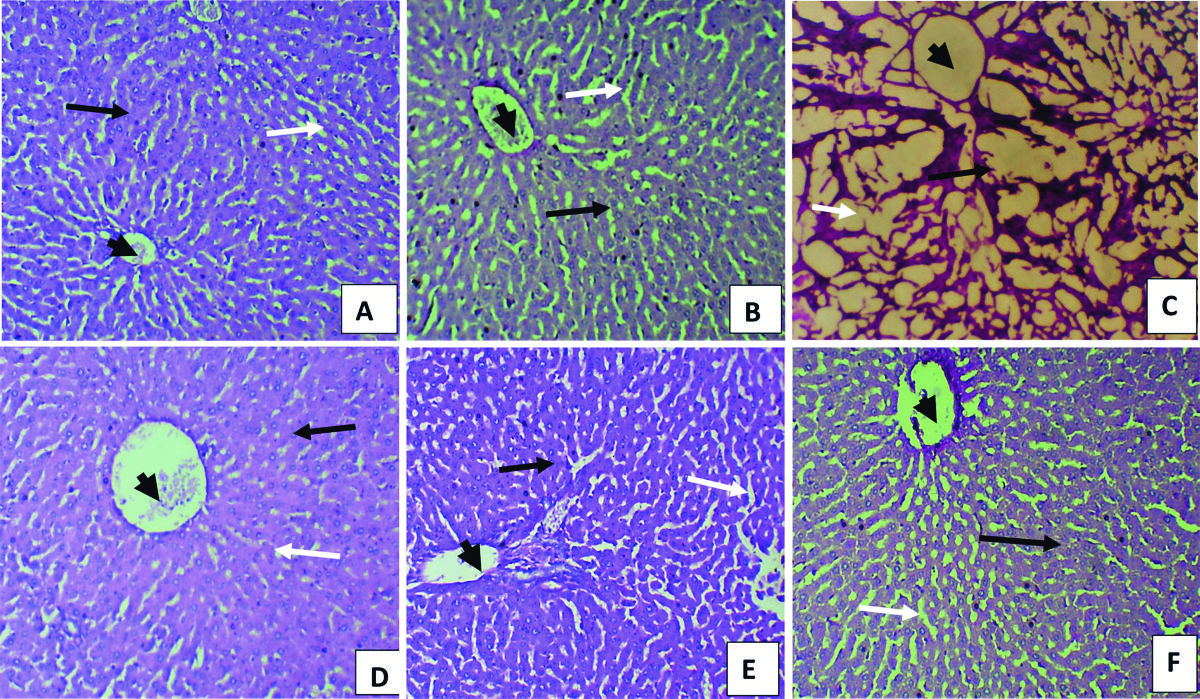
Fig. 2A-F represents the micrographs of the liver stained with PAS to show the presence of glycogen in the hepatocytes. The presence of glycogen is indicated by a magenta-colored cytoplasm and blue-black nucleus. The micrograph of the liver tissue in all groups showed the presence of abundant distribution of glycogen in the cytoplasm of the hepatocytes in all groups, which was most marked in Group III rats (Fig. 2F).
Morphometric findings of the liver
The central vein diameter in the diabetic untreated rats (group III) showed a significantly (P<0.05) wider sinusoids and central vein when compared to the extract treated (group IV and V), non-diabetic (groups I and II) and insulin-treated group (VI). The hepatocytes in the extract treated group (IV) were significantly (P<0.05) larger than the hepatocytes in other groups (Table 1).
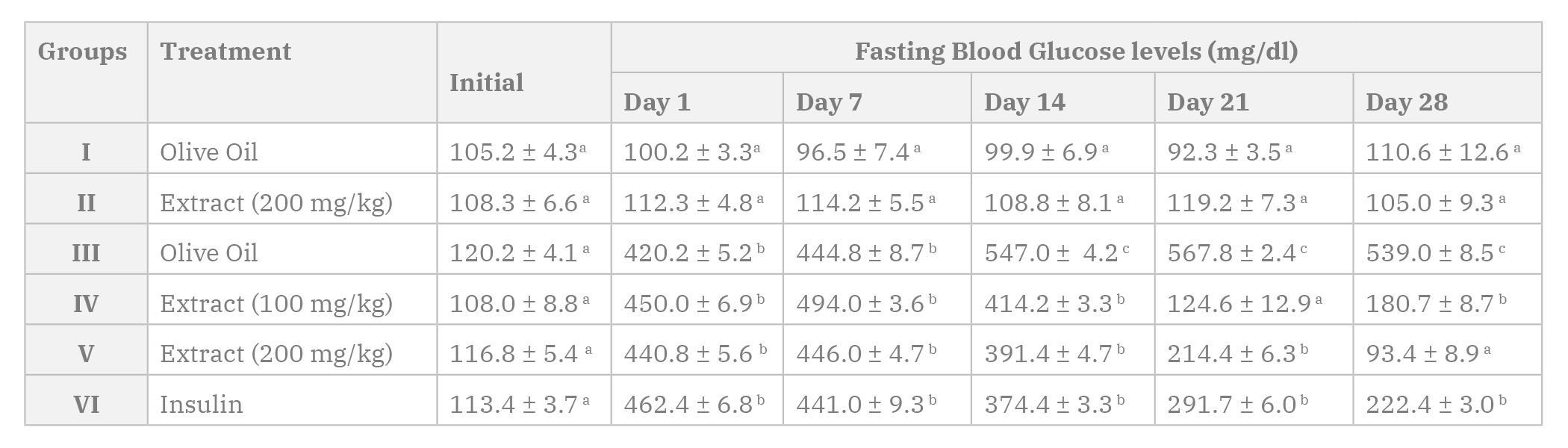
Hypoglycemic activity of Leptadenia Hastata
The hypoglycemic/anti-diabetic activity of the extract was evaluated by demonstrating the effect of the extract on fasting blood glucose, and the results obtained are presented in Table 2 below. The extract demonstrated a progressive reduction in fasting blood glucose level in diabetic rats, which became noteworthy on days 21 and 28 for animals administered with 100 mg/kg and 200 mg/kg of the extract respectively. This was comparable to the reduction observed in animals injected with insulin (days 14, 21 and 28). However, no significant reduction was observed in the fasting blood glucose in normal non-diabetic rats. Non-diabetic rats that were administered the extract in group II also showed no significant change in blood glucose levels (Table 2).
DISCUSSION
Diabetes mellitus is a metabolic disorder characterized by hyperglycaemia, which predisposes sufferers to chronic complications affecting several organs of the body, including the eye, blood vessels, kidneys, liver and nerves (Ahmed, 2005). The liver is an important organ that has its main function in maintaining and controlling blood glucose through the processes of glycogenesis and glycogenolysis. Hepatocyte damage and lipid peroxidation products induce an inflammatory response. Diabetes mellitus is considered to be one of the most common causes of liver damage (Manna et al., 2010). It has been correlated with the entire spectrum of liver diseases, including abnormal levels of liver enzymes, non-alcoholic fatty liver disease and liver cirrhosis and carcinoma (Kini et al., 2016; Al-Ani et al., 2017).
The anti-diabetic property of the extract was observed as it reduced blood glucose levels in the treated groups, while having no effect on the serum glucose levels in non-diabetic groups. The glucose-lowering property is well documented by several researchers like Bello et al. (2011), Sanda et al., (2013), Umaru (2018) and Attah (2019a) have evaluated the hypo-glycaemic and hypolipidaemic effects of water and methanol and root extracts of L. hastata in normal and alloxan-induced diabetic rat models and found out that the plant has glucose lowering properties.
In the present study, the diabetic control group showed high level of cellular abnormalities including necrosis, cellular and vascular degeneration, vascular congestion, hyperplasia of the hepatocytes and vacuolation, which are features observed in a diabetic liver. This is similar to the result obtained in a study carried out by Salih et al., 2009 who observed hydrophobic changes, necrotic aggregation and aggregation of lymphocytes in the liver of rats induced with streptozotocin. There was also a severe reduction in glycogen content of hepatic cells in the untreated diabetic groups as evidenced by the degenerated hepatocytes. Histopathological evaluation of the liver after oral ingestion of 100 mg/kg and 200 mg/kg of n-hexane extract indicated that the extract did not adversely affect the morphology of the liver, but preserved the histology of the liver to be similar to the control group. The results are similar to studies conducted by Waer and Helmy (2012), Oyebadejo et al. (2014) and Al-Ani et al. (2017), where plant extracts were observed to have ameliorated the adverse histopathological effects on the liver of diabetic rats.
The liver tissue treated with extract had significantly reduced sinusoid and central vein compared to the diabetic control group. The hepatocytes in the extract treated groups were also significantly larger than the diabetic group, and this increase may be the result of enlargement of metabolically active cells to be able to cope with the additional stress on the liver tissue.
Sinusoidal dilatation, which was present in the diabetic rats, has been suggested to represent the early stages of hyperplasia of the sinusoidal lining cells of the liver. It could be also caused by impaired portal perfusion or an inflammatory reaction. This feature is described by Marzano et al. (2015). The extract may have played a role in protecting the liver in the treated groups from an inflammatory reaction that was observable in the diabetic control group. The mechanism of the ameliorative effect of Leptadenia hastata extract on the histopathological changes in STZ- induced diabetic rats is still not clear. However, phytochemical analysis of Leptadenia hastata has revealed the presence of triterpenoids, which has been reported to be hepatoprotective (Mandal et al., 2015). Triterpenes isolated from L. hastata latex have also been known to possess anti-inflammatory activity (Nikiema et al., 2011). Zinc has been found to have insulin-like effect in that it enhances glucose uptake by inhibiting glycogen synthesis (Schlenger et al., 2014). Iron is an essential element for wide varieties of metabolic processes including playing pathogenic roles in diabetes mellitus and its complications. Some trace elements (Zn, Cr, and Mn) have important roles in metabolism and insulin action (Djama et al., 2012). These elements were found in trace amounts in the n-hexane extract of Leptadenia hastata when analysed (Attah et al., 2019b).
COMPLIANCE WITH ETHICAL STANDARDS
The experimental procedures were conducted in accordance with the University of Maiduguri Research and Ethical Committee guidelines, the ARRIVE guidelines (reporting of in vivo experiment), and the National Institutes of Health (NIH) guide for the CARE and use of laboratory animals (NIH Publications No. 8023, revised 1978). The research was also conducted in accordance with the Helsinki Declaration of 1975, as revised in 2000.
ACKNOWLEDGEMENTS
The Authors appreciate the support given by H.B. Ishaya Ph.D., N.I. Dibal, M.S. Chiroma Ph.D., J.V. Zirahei Ph.D. for the moral and academic support. The authors also appreciate the staff of the Histology Laboratory, Department of Human Anatomy, University of Maiduguri and the staff of Pharmacognosy Laboratory, Faculty of Pharmacy, University of Maiduguri for the endless hours of assistance during the experimental study.