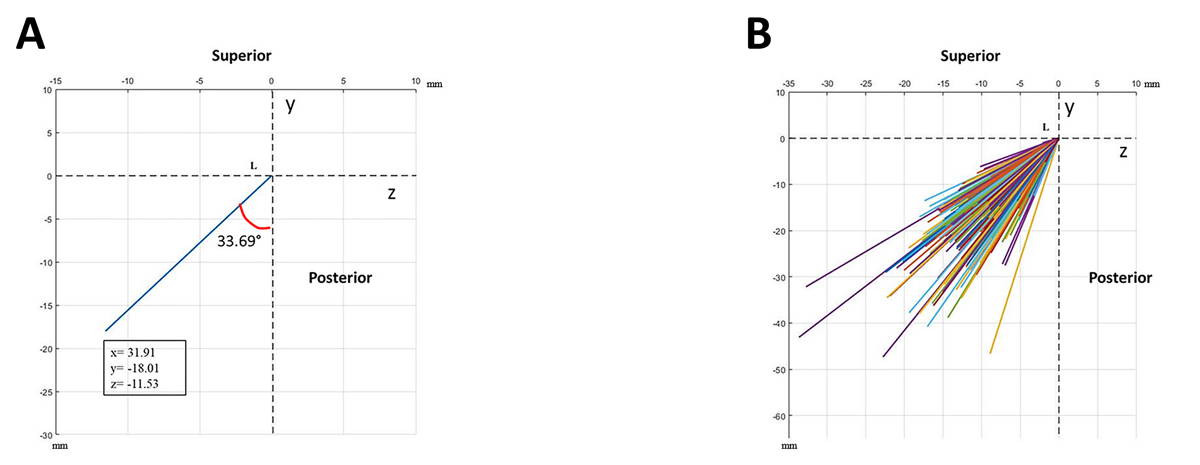
This study’s goal was to obtain the length, medial-anterior angulation, and basis-apex coordinates of the styloid process (SP) on 3D-CT images of asymptomatic individuals. In this study, the anterior and lateral lengths, anterior and medial angulation, and coordinate values on the x, y, and z-axes of the basis-apex of the SP in 259 cases (132 males, 127 females) were investigated. The mean anterior SP lengths were detected to be 23.65 mm on the right side and 23.35 mm on the left side, and the mean lateral SP lengths were detected to be 21.77 mm and 21.64 mm on the right and left sides, respectively. The mean medial angulation of the SP was measured to be 64.37° on the right side and 64.42° on the left side, and the mean anterior angulation was measured to be 30.16° and 33.69° on the right and left sides, respectively. The SP basis coordinates (right: x= -40.70, y=0, z=0, left: x=40.39, y=0, z=0) and SP apex coordinates (right: x= -31.74, y= -18.90, z= -10.36, left: x=31.91, y= -18.01, z= -11.53) were determined in mm. The results of this study will positively affect the quality of the report of radiologists who report CT images of the SP in daily routine practice. Furthermore, it is important for the prevention of complications, which may be encountered during the pre-postoperative period by otorhinolaryngologists dealing with the surgery of this region.
A different perspective on the styloid process morphometry
Ahmet Dursun1, Kenan Öztürk1, Fatih A. Şenel2, Veysel A. Ayyildiz3
1 Department of Anatomy, Faculty of Medicine, Suleyman Demirel University, Isparta, Turkey
2 Department of Computer Engineering, Engineering Faculty, Suleyman Demirel University, Isparta, Turkey
3 Department of Radiology, Faculty of Medicine, Suleyman Demirel University, Isparta, Turkey
SUMMARY
Sign up or Login
INTRODUCTION
The styloid process (SP) is a thin needle-like protrusion located in the anterior part of the stylomastoid foramen, emerging from the lower surface of the petrous part of the temporal bone, extending downward and anteriorly, and extending between the external and internal carotid arteries toward the tonsillar fossa (Kosar et al., 2011).
The SP continues to elongate as a result of ossification until the age of 8, and its elongation slows down at the age of about 30 (Baylan, 2017). The SP length may vary according to individual factors in different populations (Şener et al., 2018). However, in the literature, the normal length of this structure was reported to be between 20-30 mm. In cases with the SP length of more than 30 mm, it was called the elongated SP, and it was reported that it might cause Eagle syndrome (Şener et al., 2018). In many studies conducted to evaluate the SP, the elongated SP was reported in 4% of patients (Shayganfar et al., 2018). Of these patients, 4-10.3% were reported to be symptomatic (Shayganfar et al., 2018). In the case of elongated SP, it can cause symptoms, such as foreign body sensation in the throat, pain when moving the head, vertigo, dysphagia, otalgia, facial pain, headache, tinnitus, and trismus by creating pressure on the adjacent neurovascular structures (Hettiarachchi et al., 2019)prevalence and type of elongation, and angulation of the styloid process in relation to sex and side on digital panoramic radiographs in a Sri Lankan population. Methods: A total of 100 digital panoramic images selected from the database at the Division of Oral Medicine and Radiology, Faculty of Dental Sciences, University of Peradeniya, Sri Lanka were evaluated for the radiological features of the styloid process. Lengths greater than 30 mm were consider as elongated. Elongated styloid processes were also classified into three types based on Langlais classification (elongated, pseudo articulated; and segmented. It is possible to confuse the elongated SP with other diseases causing the same symptoms in the head and neck region. Therefore, the elongated SP is difficult to diagnose only with clinical findings and should be radiologically examined for a definitive diagnosis (Piagkou et al., 2009). It has been reported that the SP length can be evaluated in the best way upon 3D-computed tomography (CT), multi-slice CT, multi-plane reconstruction, and maximum intensity projection (Okur et al., 2014).
Cranial nerves IX, X, XI, and XII, the sympathetic trunk, the internal jugular vein, and the internal carotid artery are localized in the medial of the SP (Hettiarachchi et al., 2019)prevalence and type of elongation, and angulation of the styloid process in relation to sex and side on digital panoramic radiographs in a Sri Lankan population. Methods: A total of 100 digital panoramic images selected from the database at the Division of Oral Medicine and Radiology, Faculty of Dental Sciences, University of Peradeniya, Sri Lanka were evaluated for the radiological features of the styloid process. Lengths greater than 30 mm were consider as elongated. Elongated styloid processes were also classified into three types based on Langlais classification (elongated, pseudo articulated; and segmented. The deviation of the apex of the SP to the anterior and medial is clinically significant because it affects the adjacent neurovascular structures. Therefore, this study aimed to obtain the lengths, medial and anterior angulations, and especially the basis and apex coordinates of the SP, which had not been examined in the literature yet, in asymptomatic individuals (individuals who were thought not to have Eagle syndrome).
MATERIALS AND METHODS
This study was performed retrospectively on 3D-CT images of asymptomatic individuals (by obtaining 3D images from CT images using a RadiAnt DICOM viewer) acquired from the hospital’s “Picture Archiving and Communicating System” (PACS) in the Radiology Department. Approval was obtained from Suleyman Demirel University Faculty of Medicine Clinical Research Ethics Committee. In this study, the morphometric properties of the SP were investigated in 259 individuals (132 males, 127 females) aged between 5 and 98 years (mean age 46.25 ± 24.58).
Morphometric measurements
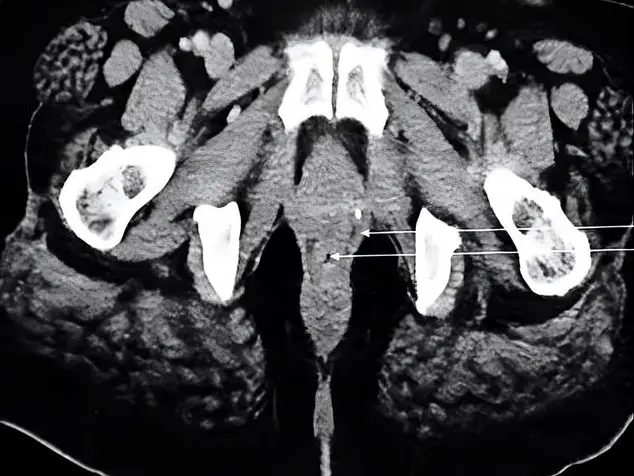
SP length: The anterior (Fig. 1) and lateral (Fig. 2) SP lengths were obtained by measuring the right and left-side SP lengths separately from the anterior and lateral sides.
Origin point: The point at which the vertical axis passing through the pharyngeal tubercle intersects the axis, which joins the SP bases, was considered to be the origin point (Fig. 1).
Apex coordinates: The coordinates of the SP apexes were determined on the coordinate plane (x, y, z).
x: On the coronal plane, it was accepted as the transverse axis, which joins the bases of the SP (Fig. 1).
y: On the coronal plane, it was accepted as the vertical axis, which passes through the pharyngeal tubercle (Fig. 1).
z: It was taken as the sagittal axis, which passes through the junction of the x and y-axes (Fig. 2).
Basis coordinates: The distances of the bases of the SP from the origin point were measured.
Medial angulation of the SP (MA): The angle between the long axis of the SP on the coronal plane and the axis joining the SP bases was measured.
Anterior angulation of the SP (AA): The angle between the axis passing through the back edge of the SP in the sagittal plane and the vertical axis passing through the base of the SP was measured (AA measurement was made in this way, as it will provide more practical use when performing radiological evaluation).
In 3D-CT images, the length measurements of the SP from the anterior and lateral parts are different from each other, because the angulations of the SP toward the anterior and medial parts are different. For both measurements to be equal to each other, the angulations of the SP toward the anterior and medial parts should be corrected to be zero. If measurements are performed by correcting these angulations, the length measurements of the SP, made from both the anterior and lateral parts, will be equal to each other. In practical use, these angulation corrections are very difficult to apply. Therefore, the SP lengths were measured from the anterior and lateral parts separately, which can be used in a radiological evaluation.
By converting the CT images of the patients into a 3D format using a “RadiAnt DICOM viewer 4.6.9”, the places to be measured were marked, and the screen image was taken and transferred to the MATLAB program. Afterward, using the ginput tool of the MATLAB program, the SP lengths, angulations, and positions on the coordinate plane were determined according to a specified reference measure. Drawings showing the position of the SP on the coordinate plane were also obtained using the MATLAB program (Figs. 3, 4, 5).
Statistical analysis
Statistical analysis was performed using SPSS Inc. for Windows 20.0 program. The arithmetic means and standard deviations of the parameters were determined according to age, side, and gender. The Kruskal-Wallis test was used to compare parameters between the age groups (decades). The Independent Samples T-Test was used to perform pairwise comparison between the genders, sides and decades. Pearson’s correlation analysis was used for the correlation analysis.
RESULTS
The SP length was measured from the anterior and lateral sides (Table 1). The SP lengths (anterior, lateral) were compared on the right and left sides according to gender, and the SP lengths on both sides were found to be more significant in male patients. However, only the right lateral SP length was statistically significantly longer in male patients (p=0.032) (Table 1). There was no statistically significant difference between the anterior and lateral SP lengths on the right and left sides (Table 1). The mean medial angulation (MA) and anterior angulation (AA) on the right and left sides were determined (Table 1, Figs. 3a, 4a, 5a). The MA and AA were compared on the right and left sides according to gender. The MA was found to be statistically significantly higher in male patients on both sides (p=0.029 on the right side and p<0.001 on the left side). The AA was also higher in male patients on both sides. However, it was not statistically significant (Table 1). The MA and AA on the right and left sides were compared regardless of gender. Only the AA was found to be statistically significantly higher on the left side (p<0.001) (Table 1).
The mean coordinate values of the bases and apexes of the SPs were determined (Table 1). All SPs were displayed from the anterior (Fig. 3b) and lateral (Figs. 4b, 5b). The mean coordinate values of the bases and apexes in the anterior and lateral views of the SPs and the MA and AA values were displayed on the coordinate plane (Figs. 3a, 4a, 5a).
The mean values of the anterior and lateral SP lengths, MA and AA according to decades were obtained, and it was determined whether there was a statistically significant difference between the decades (Table 2). The MA did not differ statistically significantly between the decades on both sides (Table 2). While the AA on the right side did not differ statistically significantly between the decades, it differed statistically significantly between the decades on the left side AA. When a pairwise comparison was made between the decades, it was observed that this difference originated from the 3rd and 8th decades. A statistically significant difference was observed between the decades in all parameters related to the SP length (p<0.05). This difference originated from the 1st decade in all parameters (Table 2).
DISCUSSION
Eagle (1937) reported the normal length of the SP to be between 25-30 mm and accepted the SP longer than 30 mm as elongated. Eagle (1937) also stated that the elongated SP might cause Eagle syndrome. Jung et al. (2004)based on early reports, do not respect the natural variation of the SP. The aim of this study is to investigate the natural variation of the length of the SP. Knowing this variation is a prerequisite for consistent terminology in anatomy and anthropology; it is essential for the classification of the SP as elongated on panoramic radiographs (PRs accepted the SP to be elongated if it was longer than 45 mm. The most prominent symptom of Eagle syndrome is chronic pain in the head and neck region, which is caused by the elongated SP creating pressure on the adjacent structures. However, despite the elongated SP in many patients, its symptoms were not encountered (Burulday et al., 2017; Hettiarachchi et al., 2019; Kosar et al., 2011; Okur et al., 2014)which is an affective modality in the identification of ES, and a comparison with related studies. Three-dimensional (3D.
Knowing the SP length is important for the diagnosis of Eagle syndrome. However, it is not sufficient to know only the SP length, because the deviations of the SP also lead to Eagle syndrome by affecting the adjacent structures. It has been reported that although the SP has normal dimensions, it can be palpated in the tonsillar fossa as a result of its medial deviation (Frommer, 1974). Furthermore, lateral deviation causes compression in the external carotid artery, and posterior deviation causes compression in cranial nerves IX, X, XI, and XII. Anterior deviation irritates the mucosa and creates pressure on vital structures in the tonsillar region (Mazzetto et al., 2013; Piagkou et al., 2009). Therefore, studies investigating the medial and anterior angulation of the SP in addition to its length have been conducted in asymptomatic individuals (Burulday et al., 2017; Kosar et al., 2011). In the present study, the coordinates of the basis and apex of the SP were determined, in addition to examining the above-mentioned parameters. These coordinates will demonstrate with which structures the apex of the SP is closely related, and will provide more detailed information about the probability of its influencing the adjacent structures.
Multi-slice CT and 3D-CT are among the best imaging modalities to evaluate the SP length (Okur et al., 2014). 3D-CT was reported to be the most reliable imaging method to determine the SP length and MA, and to investigate the relationship between the SP and adjacent structures (Pokharel et al., 2013). Considering that there are clinicians using imaging methods other than 3D-CT, it is thought to be more useful to measure the SP length separately from the anterior and lateral sides.
Ekici et al. (2013) and the distribution of the SP lengths in different age and sex groups using MDCT. MDCT scans were performed in 805 patients (401 males, 404 females found a higher mean SP length in males than in females (p<0.001) (Table 3). They determined the mean SP length to be 30.7 mm in the 4th decade, 32.9 mm in the 5th decade, 32.1 mm in the 6th decade, and 32.6 mm in the 7th decade. Cullu et al. (2013) found the SP length in multidetector computed tomography (MDCT) images to be 28.5 mm in the 4th decade and 28.2 mm in the 5th decade. The results of the present study are smaller than the results obtained by Ekici et al. (2013)and the distribution of the SP lengths in different age and sex groups using MDCT. MDCT scans were performed in 805 patients (401 males, 404 females and Cullu et al. (2013). This situation may be due to different imaging methods and measurement locations.
The results obtained by Custodio et al. (2016) regarding the lateral SP length in the skull are presented in Table 3. The results of the current study regarding the lateral SP length are consistent with the above-mentioned lateral measurement results. This may be since 3D-CT imaging is the imaging method closest to reality. Shayganfar et al. (2018) reported in their study that the SP length varied between 4.1-57 mm on the right side and 7-62 mm on the left side (age range 12-75). In our study, the SP length was determined to be between 2.12-61.02 mm on the right side and 2.35-60.65 mm on the left side (age range 5-98). The reason for this small difference between the study carried out by Shayganfar et al. (2018) and our results may be that the samples in our study started from a younger age.
In Table 3, differences between the results of the studies conducted on the SP length in the Turkish population suggest that the effect of ethnicity on the SP length is weak, although measurement and imaging methods are different. Steinmann (1968) suggested three different theories that attempted to explain the cause of the elongated SP. The first one is the “Reactive Hyperplasia Theory”, in which the post-traumatic stylohyoid ligament continues to ossify from the end of the SP. The second theory is the “Reactive Metaplasia Theory”, in which the stylohyoid ligament ossifies with a traumatic stimulus. The second theory is explained by the presence of ossification centers in the stylohyoid ligament. These two theories assert the ossification of the stylohyoid ligament after a traumatic event in any age group. The third theory was suggested as a simple anatomical variation with normal ossification without any trauma. This anatomic variation explains the ossification observed in children and young people who have not experienced any trauma. Camarda et al. (1989) explained the ossification observed in adults due to aging without trauma by the “Theory of Aging Developmental Anomaly”.
In previous studies (Balcioglu et al., 2009; Buyuk et al., 2018; Shayganfar et al., 2018), similarly to our study, the anterior SP length was found to be longer in male patients than in female patients (Table 3). While no statistically significant difference was observed between genders in the present study, a significant difference was reported in the studies conducted by Büyük et al. (2018), Balcıoğlu et al. (2009), and Shayganfar et al. (2018). In our study, no statistically significant difference was observed between the anterior and lateral SP lengths on the right and left sides. Balcıoğlu et al. (2009) and Burulday et al. (2017) indicated that there was no statistically significant difference between the SP lengths on both sides, similarly to our study. The anterior and lateral SP lengths differed statistically significantly on both sides between decades (Table 2). When a pairwise comparison was made between decades, it was determined that this difference originated from the 1st decade. Although the mean in the 3rd decade was higher than that in the 2nd decade, there was no statistically significant difference between them. This situation suggests that the SP development was very fast in the 1st decade, slowed down in the 2nd decade and completed in the 3rd decade.
According to Table 3, the MA results of this study are consistent only with the results obtained by Burulday et al. (2017). Although the method employed was the same, the results obtained by Kent et al. (2015) were different from the MA results of the present study. The reason for this difference may be that the population studied was different. Although Büyük et al. (2018) and Ekici et al. (2013) conducted research with the same population as in our study, they employed different imaging methods such as cone beam computed tomography (CBCT) and MDCT, respectively. This may be the reason why the MA results of the present study differ from the results obtained by Büyük et al. (2018) and Ekici et al. (2013).
The AA acquired by Kent et al. (2015) was smaller than that in our study (Table 3). This situation may be due to a different ethnic origin. The reason for the fact that the AA found in our study was different from the AA obtained by Yavuz et al. (2008) in Table 3 may be the use of a different imaging method (Towne’s X-ray) and the use of different reference lines when measuring the angulation.
It is thought that the difference between the 3rd and 8th decades in the left AA is due to the randomly collected samples evaluated in these decades. The fact that the difference in the left AA did not originate from the 1st decade (the difference in lengths originated from the 1st decade) indicates that the anterior and medial angulations did not change statistically significantly from the 1st decade.
In the study performed by Ayyıldız et al. (2019) on Eagle syndrome patients, the imaging method and measurement methods used were the same as in our study. Upon comparing the above-mentioned results with the results of our study in asymptomatic individuals, the results of the SP anterior and medial angulation were similar. However, the results of the SP anterior and lateral lengths were different (Table 3). Therefore, it is thought that the anterior and medial angulation alone cannot be sufficient for the diagnosis of Eagle syndrome, and it may be more useful to know the SP length and coordinates.
In conclusion, the fact that the present study was conducted using the 3D-CT imaging method will provide detailed and reliable information about the SP length, angulation, and coordinates. The results of this study on the SP will help clinicians dealing with the SP in determining the variational status of the SP more easily. Knowing the length, angulation, and coordinates of the SP in asymptomatic individuals will positively affect the quality of the report of radiologists who report CT images of this region in daily routine practice. Furthermore, it is important for the prevention of complications, which may be encountered during the pre-postoperative period by otorhinolaryngologists dealing with the surgery of this region, and for the guidance of the surgery.
Related articles
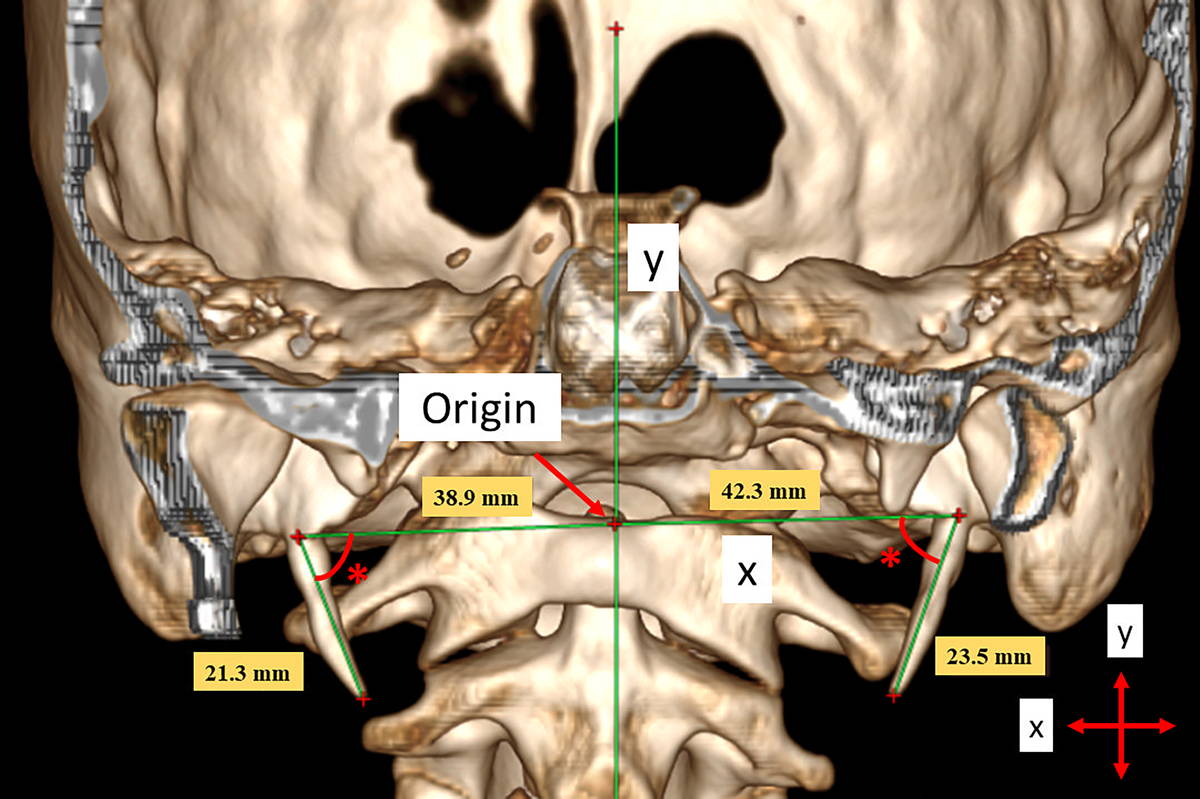 Fig. 1.- A 19-year-old male patient. The SP lengths measured from the anterior view and the x- and y-coordinate planes. (*) Medial angulation.
Fig. 1.- A 19-year-old male patient. The SP lengths measured from the anterior view and the x- and y-coordinate planes. (*) Medial angulation.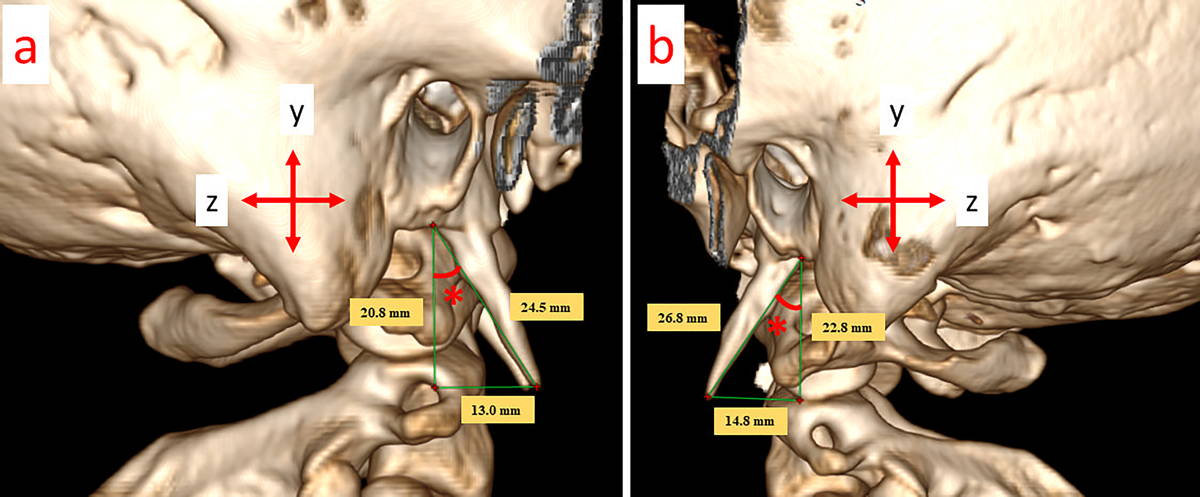 Fig. 2.- A 19-year-old male patient (The same patient as in Fig. 1). The SP lengths measured from the lateral (a. right, b. left) view and the y- and z-coordinate planes; (*) Anterior angulation.
Fig. 2.- A 19-year-old male patient (The same patient as in Fig. 1). The SP lengths measured from the lateral (a. right, b. left) view and the y- and z-coordinate planes; (*) Anterior angulation.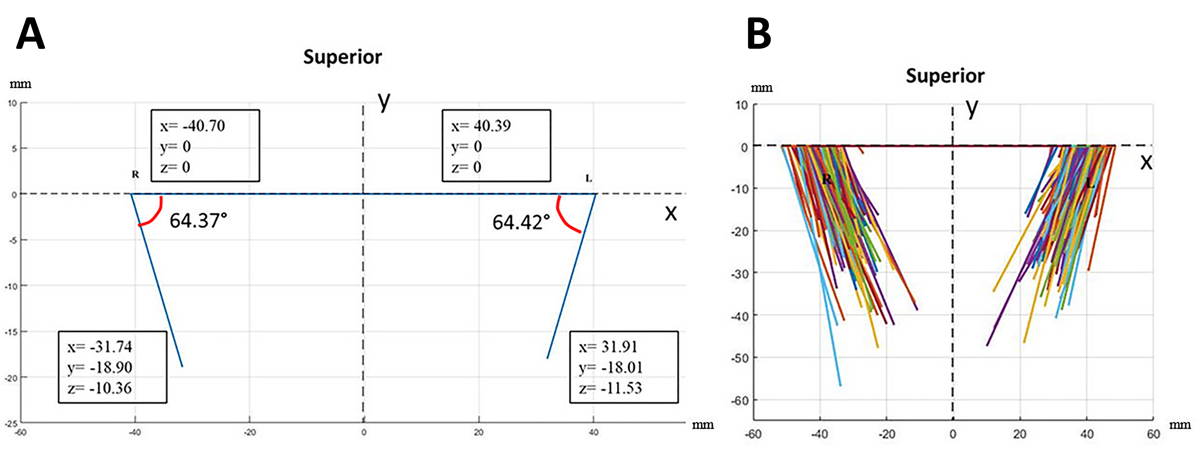 Fig. 3.- a. The mean medial angulations and the mean coordinate values of the base and apex of the SP when viewed from the anterior part; b. The anterior views of all SPs.
Fig. 3.- a. The mean medial angulations and the mean coordinate values of the base and apex of the SP when viewed from the anterior part; b. The anterior views of all SPs. Fig. 4.- a. The mean anterior angulation and the mean coordinate values of the apex of the SP when viewed at the right SP from the lateral part; b. The lateral views of all the right SPs.
Fig. 4.- a. The mean anterior angulation and the mean coordinate values of the apex of the SP when viewed at the right SP from the lateral part; b. The lateral views of all the right SPs.AYYILDIZ VA, SENEL FA, DURSUN A, OZTURK K (2019) Morphometric examination of the styloid process by 3D-CT in patients with Eagle syndrome. Eur Arch Oto-Rhino-Laryngology, 276: 3453-3459.
BALCIOGLU HA, KILIC C, AKYOL M, OZAN H, KOKTEN G (2009) Length of the styloid process and anatomical implications for Eagle’s syndrome. Folia Morphol (Warsz), 68: 265-270.
BAYLAN H (2017) The anatomical basis of the symptoms of an elongated styloid process. J Hum Rhythm, 3: 32-35.
BURULDAY V, AKGUL MH, BAYAR MULUK N, YAGDIRAN B, INAL M (2017) The importance of medial-lateral styloid process angulation/coronal plane angle in symptomatic Eagle syndrome. Clin Anat, 30: 487-491.
BUYUK C, GUNDUZ K, AVSEVER H (2018) Morphological assessment of the stylohyoid complex variations with cone beam computed tomography in a Turkish population. Folia Morphol (Warsz), 77: 79-89.
CAMARDA AJ, DESCHAMPS C, FOREST DI (1989) Stylohyoid chain ossification: A discussion of etiology. Oral Surg Oral Med Oral Pathol, 67: 508-514.
CULLU N, DEVEER M, SAHAN M, TETIKER H, YILMAZ M (2013) Radiological evaluation of the styloid process length in the normal population. Folia Morphol, 72: 318-321.
CUSTODIO ALN, SILVA MRA, ABREU MH, ARAÚJO LRA, DE OLIVEIRA LJ (2016) Styloid process of the temporal bone: morphometric analysis and clinical ımplications. Biomed Res Int, 2016: 1-5.
EAGLE W (1937) Elongated styloid process. Report of two cases. Arch Otolaryngol, 25: 584-587.
EKICI F, TEKBAS G, HAMIDI C, ONDER H, GOYA C, CETINCAKMAK MG, GUMUS H, UYAR A, BILICI A (2013) The distribution of stylohyoid chain anatomic variations by age groups and gender: An analysis using MDCT. Eur Arch Oto-Rhino-Laryngology, 270: 1715-1720.
FROMMER J (1974) Anatomic variations in the stylohyoid chain and their possible clinical significance. Oral Surg Oral Med Oral Pathol, 38: 659-667.
HETTIARACHCHI PVKS, JAYASINGHE RM, FONSEKA MC, JAYASINGHE RD, NANAYAKKARA CD (2019) Evaluation of the styloid process in a Sri Lankan population using digital panoramic radiographs. J Oral Biol Craniofacial Res, 9: 73-76.
JUNG T, TSCHERNITSCHEK H, HIPPEN H, SCHNEIDER B, BORCHERS L (2004) Elongated styloid process: When is it really elongated? Dentomaxillofacial Radiol, 33: 119-124.
KENT DT, RATH TJ, SNYDERMAN C (2015) Conventional and 3-dimensional computerized tomography in Eagle’s syndrome, glossopharyngeal neuralgia, and asymptomatic controls. Otolaryngol - Head Neck Surg, 153: 41-47.
KOSAR MI, ATALAR MH, SABANCIOGULLARI V, TETIKER H, ERDIL FH, CIMEN M, OTAG I (2011) Evaluation of the length and angulation of the styloid process in the patient with pre-diagnosis of Eagle syndrome. Folia Morphol (Warsz), 70: 295-299.
MAZZETTO MO, ANDRADE KMD, MAGRI LV, RODRIGUES CA, WATANABE PCA (2013) Anterior and medial angulations of the styloid process in subjects with TMD: Clinical and radiographic findings. Braz Dent J, 24: 80-84.
OKUR A, OZKIRIS M, SERIN HI, GENCER ZK, KARACAVUS S, KARACA L, KANTARCI M, SAYDAM L (2014) Is there a relationship between symptoms of patients and tomographic characteristics of styloid process? Surg Radiol Anat, 36: 627-632.
PIAGKOU MN, ANAGNOSTOPOULOU S, KOULADOUROS K, PIAGKOS G (2009) Eagle’s syndrome: A review of the literature. Clin Anat, 22: 545-558.
POKHAREL M, KARKI S, SHRESTHA I, SHRESTHA BL, KHANAL K, AMATYA RCM (2013) Clinicoradiologic evaluation of Eagle’s syndrome and its management. Kathmandu Univ Med J, 11: 305-309.
SENER E, GURHAN C, CEYLAN N, GUNERI P (2018) Elongation or angulation of styloid process: discussion with a case report and review of the literature. Cumhur Dent J, 21: 396-403.
SHAYGANFAR A, GOLBIDI D, YAHAY M, NOURI S, SIRUS S (2018) Radiological evaluation of the styloid process length using 64-row multidetector computed tomography scan. Adv Biomed Res, 7: 85.
STEINMANN EP (1968) Styloid syndrome in absence of an elongated process. Acta Otolaryngol, 66: 347-356.
VADGAONKAR R, MURLIMANJU BV, PRABHU LV, RAI R, PAI MM, TONSE M, JIJI PJ (2015) Morphological study of styloid process of the temporal bone and its clinical implications. Anat Cell Biol, 48: 195-200.
YAVUZ H, CAYLAKLI F, YILDIRIM T, OZLUOGLU LN (2008) Angulation of the styloid process in Eagle’s syndrome. Eur Arch Oto-Rhino-Laryngology, 265: 1393-1396.