Decreasing estrogen levels are associated with an increased risk of dementia and Alzheimer’s disease (AD) in postmenopausal women. We used ovariectomized (OVX) rats as a model for postmenopausal women. Our studies showed that a high daily dose of 100 mL/kg body weight of young coconut juice (YCJ) helped prevent AD pathologies. We investigated the optimal dose for the neuroprotective effects of YCJ that would have least side effects following prolonged consumption and the neuroprotective effects of the YCJ acted via choline acetyltransferase (ChAT) and N-methyl-D-aspartate receptors (NMDAR1). Our results showed that rats receiving YCJ at a dose of 10 mL/kg BW/d had the least side effects in their liver and kidney. The ovariectomized rats that were given 10 mL/kg BW/d (OY10) and 20 mL/kg BW/d (OY20) of YCJ demonstrated significantly (p<0.05) higher vaginal scores, as compared to the control ovariectomized (OW) group, showed proestrus and estrus stages continuously throughout the ten weeks. A significant (p<0.05) increase was observed in the number of ChAT-ir, NMDAR1-ir, ERα-ir, and ERβ-ir pyramidal neurons in CA1, CA2 and CA3 hippocampal areas (HP) and prefrontal cortex (PF) brain regions following administration of YCJ. Moreover, there was a significant positive correlation among those number of ChAT-ir, NMDAR1-ir, ERα-ir, and ERβ-ir neurons. Our study indicated that YCJ at the lowest dose of 10 mL/kg BW/day has estrogenic effects using primarily screening vaginal smear, a test of epithelial cell changes in response to alterations in the concentration of the ovarian hormone. The mechanism of YCJ on preserving neuronal cells and prevent Alzheimer’s pathologies, ChAT, and NMDAR1 antibodies were also investigated. Estrogenic effects of YCJ were also confirmed using estrogen receptor (ERα and ERβ) antibodies. In brief, YCJ has neuroprotective effects by preserving ChAT-ir and NMDAR1-ir neurons via ERα and ERβ.
Young coconut juice prevents neuronal cell death via ChAT, NMDAR1, and estrogen receptors in the hippocampus and prefrontal cortex of ovariectomized rats
Kolip Payanglee1, Albert M. Hutapea2, Nisaudah Radenahmad1
1 Division of Health and Applied Sciences, Faculty of Science, Prince of Songkla University, Hat Yai, Songkhla, Thailand
2 Department of Pharmacy, Faculty of Science, Universitas Advent Indonesia, Bandung, Indonesia
SUMMARY
Sign up or Login
INTRODUCTION
Dementia is a significant cognitive impairment, and Alzheimer’s disease (AD) is one of the leading causes of disability in postmenopausal women, a period when circulating estrogen is reduced (van Dijk et al., 2015). AD is a neurodegenerative disorder of the brain that progresses slowly characterized by the formation of amyloid beta (Aβ) and neurofibrillary tangles (Sezgin and Dincer, 2014).
Acetylcholine modulates cognitive performances, learning, and memory processes. Impaired cortical cholinergic neurotransmission may also contribute to Aβ plaque and neurofibrillary tangles in AD (Ferreira-Vieira et al., 2016). Several studies reveal that ovariectomy induces pathology of AD and causes changes in cholinergic systems. Schliebs and Arendt (2006) found a correlation between clinical dementia ratings and reductions in the numbers of cortical cholinergic markers such as choline acetyltransferase (ChAT), muscarinic and nicotinic acetylcholine receptor binding sites, as well as in the levels of acetylcholine. Carroll et al. (2007) found that ovariectomy-induced depletion of sex steroid hormones in adult female 3xTg-AD mice significantly increased amyloid-beta accumulation and worsened memory performance. However, treatment of these ovariectomized mice with estrogen, but not progesterone, prevented these effects. According to Szego et al. (2011), estrogen-pretreatment on Aβ-induced cholinergic neurodegeneration in the nucleus basalis magnocellularis (NBM) decreased cholinergic neuron loss and partly prevented fiber degeneration. Another group of researchers found that hormone replacement therapy (HRT) in OVX rats increased ChAT protein levels in the hippocampus (HP) and prefrontal cortex (PF) (Bohacek et al., 2008).
The short-term release of glutamate is involved in learning and memory (Collingridge and Singer, 1990). Glutamatergic neurotransmission in the neocortical regions and the hippocampus is severely disrupted in AD (Greenamyre, 1986; Maragos et al., 1987; Palmer and Gershon, 1990). It has been reported that an Aβ peptide blocks glutamate uptake, thus induces an increase of extracellular glutamate (Fernandez-Tome et al., 2004). Consequently, the extracellular glutamate level elevation induces excessive over stimulation of the N-methyl-D-aspartate receptors (NMDAR), which can trigger an intracellular Ca2+ influx leading to neuronal cell death (Lauderback et al., 2001).
The use of estrogen-based HRT in postmenopausal women can reduce the risk of AD but can also increase the risk of certain diseases like breast cancer (Bitzer et al., 2008), ovarian cancer (Zhou et al., 2008), and endometrial cancer (McCullough, 2008). Therefore, phytoestrogen could be a candidate for a safer alternative for exogenous estrogen in replacing HRT. Our previous studies have shown that young coconut juice (YCJ) (Cocos nucifera L., Arecaceae), known to contain β-sitosterol (Rattanaburee et al., 2014) delayed AD pathologies (Radenahmad et al., 2011) and prevented neuronal cell death (Radenahmad et al., 2009; Payanglee et al., 2017). In the present study, we further investigated the neuroprotective effects of YCJ in preserving neuronal cells via ChAT and NMDAR1 that represent the cholinergic and glutamatergic systems in the HP and PF. These areas are involved in cognitive function and spatial memory. Besides, we also investigated the optimal neuroprotective dose of YCJ that would cause the least side effects following its prolonged consumption by lowering the concentration of YCJ from 100 mL/kg BW to 10 mL/kg BW, 20 mL/kg BW, and 40 mL/kg BW.
MATERIALS AND METHODS
YCJ preparation
A large volume of YCJ was collected from Khlong Hoi Khong district, Hat Yai, Songkhla, Thailand. It was then dried, and the powder formed was kept at -30°C until used. The powder was freshly reconstituted and prepared daily for the oral intake. A complete description of YCJ, including its preparation and administration, is provided in our previous publication (Radenahmad et al., 2006).
Animals
Adult female Wistar rats (8-month-old and 250-300 g bw) were purchased from Mahidol University, Salaya campus. The animals were maintained on standard food pellet housed in a room free from any source of chemical contamination, artificially illuminated (12h dark/light cycle) and thermally controlled (25 ± 1°C) and humidity (50 ± 5%) at the Animal House Laboratory, Faculty of Science, Prince of Songkla University, Hat Yai, Songkhla, Thailand. All animals were received humane care in compliance with the guidelines of the Animal Care and Use Committee of Prince of Songkla University and the National Institutes of Health (NIH publication 86-23 revised 1985. The protocol was approved under the license number 04/57).
Experimental design
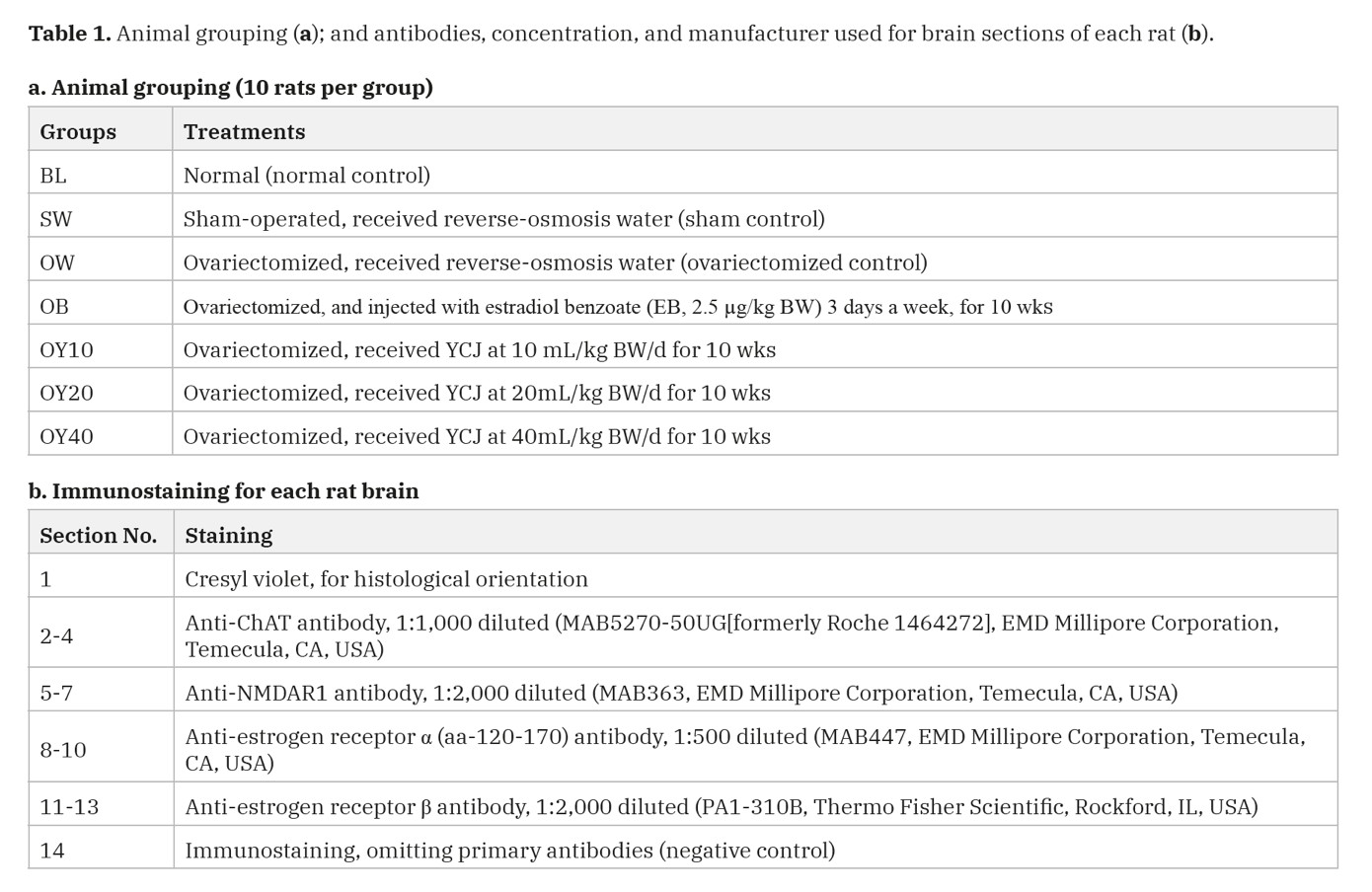
A total of seventy rats were randomly divided into seven groups (Table 1a), with ten rats per group such as the first group, the baseline (BL); second, the sham-operated (SW); third, the OVX rats (OW); and the fourth, the OVX rats injected with exogenous estrogen (2.5 µg/kg BW), a dose of estradiol benzoate for three days a week (OB) given was the same as in the previous studies (Radenahmad et al., 2009; 2011). The fifth, sixth, and seventh groups consisted of OVX rats daily received 10 (OY10), 20 (OY20), and 40 (OY40) mL/kg BW/day of YCJ, respectively. The administration of EB and YCJ started one week after the ovariectomy was performed. SW and OW rats were forced-fed with injection vehicle (reverse osmosis water) instead. YCJ were treated once a day every day, began one week following ovariectomy. After ten weeks of feeding and injection treatment, the rats were sacrificed; the brains were fixed with 10% neutral formalin, processed through paraffin sectioning and immunohistochemical staining. Serum was collected for estradiol measurements using the chemiluminescent immunoassay (CIA) technique (ECLIA, Modular E 170C, Estradiol II 03000079 122, Roche, Germany).
Evaluation of the estrous cycle
Examining the effect of the estrogen-like-compound inYCJ on the vagina was performed using vaginal smears (Marcondes, 2002). Vaginal secretions were collected with a plastic pipette filled with 10 µL of normal saline. The samples containing cells were placed on microscopic glass slides and air-dried prior to staining and then stained with the Papanicolaou (Pap) stain before being examined with a light microscope. Scoring of vaginal smears was performed as follows:
|
Estrous cycle |
Scores |
|
Proestrus |
3 |
|
Estrus |
2 |
|
Metestrus |
1 |
|
Diestrus |
1 |
Immunohistochemistry
Fourteen 5-µm-thick sections collected from each block were prepared for cresyl violet stain and immunostaining (Table 1b). For immunostaining, the glass slides were coated by poly-L-lysine solution. Sections of uterus and ovary from normal female rats were used as positive controls for ERα, and ERβ immunostaining, respectively, and the staining process was performed according to the method previously described (Radenahmad et al., 2009; 2011; 2012). Please see the details of all antibodies, concentration, and manufacturer used for brain sections of each rat in Table 1b.
Quantitative analysis of immunoreactive cells
The total number of immunoreactive cells from the prefrontal cortex (PF) and the hippocampus (CA1, CA2, and CA3) were counted under light microscopy (LM) with 40x magnification power. The counting was performed by two blinded observers on each slide’s ten random fields using an image analysis system (Samba microscopic image processor; Samba Technologies, Meylan, France). Readings from three sections pertaining to each antibody were averaged and expressed as the mean number of immunoreactive cells/mm2.
Statistical analysis
Shapiro-Wilk test was applied to test the normal distribution. Statistical analysis was performed using the One-way ANOVA followed by LSD test available in the statistical program SPSS version 16.0 (SPSS, Inc., Chicago, IL, USA). Altman’s nomogram was used for calculations of sample size. Random selection of the microscopic fields was achieved using a computer-generated list of random numbers (Excel version 5.0). Results were expressed as mean ± SEM, and p<0.05 was considered significant.
RESULTS
Vaginal smear
The proestrus smear had a predominance of nucleated epithelial cells with a blue-purple stained nucleus and light pink stained cytoplasm (Fig. 1A, proestrus). A predominance of cornified cells was found in estrous smear (Fig. 1A, estrus). The metestrus smear showed densely packed leukocytes with blue-purple stained nucleus and a small cytoplasm and a few cornified cells (Fig. 1A, metestrus). The diestrus smear had only scattered nucleated epithelial cells consisting almost entirely of leukocytes (Fig. 1A, Diestrus).
As determined by vaginal smear cytology, all rats of the sham group (SW) exhibited four stages of the estrous cycle with an average score of 2.00±0.05, which was significantly higher than that of OW (1.17±0.02), OB (1.58±0.06), OY10 (1.24±0.03), OY20 (1.33±0.04) and OY40 (1.17±0.04) at p<0.05.
As determined by vaginal smear cytology, all rats exhibited four stages of the estrous cycle except the ovx rats (OW group), which was persistently in metestrus/diestrus. The average score of the SW group = 2.00±0.05, which was significantly higher than that of OW (1.17±0.02), OB (1.58±0.06), OY10 (1.24±0.03), OY20 (1.33±0.04), and OY40 (1.17±0.04) at p<0.05.
In summary, the average for the vaginal smear scoring of the estrus cycle for 70 days of the SW group was significantly (p<0.05) the highest, as compared to other groups (Figs. 1A, 1B). The OW group scores were significantly (p<0.05) lower compared to the OB, OY10, and OY20 groups. Vaginal smear scoring of the OB group was significantly (p<0.05) higher compared to the OY10 and OY40 groups. Surprisingly, the OY40 group scores were significantly (p<0.05) lower than those of the OY10 and OY20 groups.
Serum E2 level
The serum E2 level of the BL group was significantly (p<0.05) higher when compared to all the other groups, except for the SW group (Fig. 2). Surprisingly, serum E2 levels of the OW, OB, and OY10 were not significantly different from those of the SW group because of the SW group’s high SEM bar. Furthermore, E2 levels of the OY20 and OY40 groups were significantly lower than that of the SW group.
Histomorphometry of immunoreactive (-ir) cells
Immunoreactivity of the anti-ChAT, anti-NMDAR1, anti-ERα, and anti-ERβ antibodies was detected in the cell bodies’ cytoplasm, dendrites, and axons of the pyramidal neurons in all the seven groups examined for CA1, CA2, CA3, and PF (Figs. 3, 5, 6). The restoration of the serum E2 level following the oral intake of YCJ at 10 mL/kg BW (OY10), as demonstrated in Fig. 2, was associated with significant preservation in the neuronal cells compared to the OVX group, which did not receive any treatment. This condition was indicated by the significantly (p<0.05) higher number of neurons that were positive for ChAT, NMDAR1, ERα, and ERβ (Figs. 3 and 6). Interestingly, for some brain regions like CA1 in Fig. 3A, and PF in Fig. 3C, the number of these neurons of at least one group of YCJ treatment was also significantly (p<0.05) higher compared to the OB group.
After 70 days of treatment, when the numbers of ERα-ir and ERβ-ir cells were statistically correlated with that of ChAT with regression, an equation for ERα: y=0.522x+6.044 (p = .000) and for ERβ: y=0.411x+5.569 (p = .000), respectively, leading to the conclusion that the numbers of ChAT-ir neurons increased by way of ERα and ERβ activation (Fig. 4A).
Like ChAT, the regression equation for ERα: y =0.445x+8.109 (p = .000) and for ERβ: y=0.414x+6.269 (p = .000) leading to the conclusion that the numbers of NMDAR1-ir neurons increased by way of ERα and ERβ activation (Fig. 4B).
Furthermore, when the number of ChAT-ir neurons was correlated with that of NMDAR1-ir neurons, the p-value of the testing regression equation indicates p = .000, leading to the conclusion that these two types of neurons somehow have colocalized activity (Fig. 4C).
DISCUSSION
Alzheimer’s disease (AD) is the most common neurodegenerative disease, accounting for more than 50% of all dementia types (Plassman et al., 2007). Currently, no treatments are available to stop, slow, or reverse the progression of the disease process (Master et al., 2015). The neuroprotective role of estrogen-containing hormone therapy is useful but controversial. The primary concern with estrogen therapy as hormone replacement therapy (HRT) is increased risk of venous thrombosis, coronary artery disease, breast and endometrial carcinoma, dysmenorrhea, abnormal vaginal bleeding and hypersensitivity (Jamshed et al., 2014). Nowadays, plant-derived estrogen researches were explored to replace HRT. In previous work, we reported that YCJ at 100 mL/kg BW help halting AD pathologies (Radenahmad et al., 2009; 2011), but undesirable effects such as glycogen deposition in the liver occurred. Therefore, the lower doses of YCJ were investigated in this study to minimize the unfavorable side effects. A vaginal smear was applied to preliminary screening in living rats to ensure that such a lower dose of YCJ still had estrogenic activity,
The rat vaginal wall provides an excellent model to determine the estrogenic activity of estrogenic substances and is recognized as a simple, sensitive, and inexpensive method (Parhizkar et al., 2011). The estrogenic-like compounds have been shown to affect the differentiation of the vaginal epithelium and enhance vaginal cornification (Laws et al., 2000; Burton and Wells, 2002). The present experiment confirmed the presence of a menopausal stage in OVX rats by monitoring the cellular differentiation of the vaginal epithelium for ten consecutive weeks. In the OVX group, the vaginal smear scores were low compared to the sham control rats. The OVX rats had diestrus and metestrus stages continuously throughout the 10-week period. The ovariectomized rats that fed with 10 mL/kg BW/d (OY10) and 20 mL/kg BW/d (OY20) of YCJ showed proestrus and estrus stages continuously throughout ten weeks treatment with higher vaginal scores at p<0.05 compared to the control ovariectomized (OW) group. Nevertheless, the vaginal smear scores of OY10 and OY20 groups were significantly lower compared to the OB. During proestrus, progesterone declines, and a preovulatory follicle undergoes its final growth phase (estradiol increases). Proestrus and estrus stages comprise the follicular phase indicating a high level of estrogen. Ovulation usually occurs during estrus. While metestrus and diestrus make up the luteal phase when the estrogen drops to a very low level (Jin et al., 2018). That vaginal smear of the OY40 group was not significantly different from the ovx (OW) group, and it was significantly lower than that of the SW, OB, OY10, and OY20 groups. That vaginal smear score of the OY40 group was in the metestrus cycle, indicating a deficient estrogen level.
We have previously demonstrated the effect of estrogen-like activities of YCJ in delaying AD pathologies, protecting neuronal cell death, accelerating wound healing, and preventing bone loss (Radenahmad et al., 2006; 2009; 2011; 2012; 2014; 2015; Yusuh et al., 2010; Suwanpal et al., 2011). In some of these studies, pyramidal neurons that use excitatory neurotransmitters, and non-pyramidal ones that use inhibitory neurotransmitters, were examined using antibodies against NF200 and parvalbumin (PV), respectively. More parameters were investigated using antibodies against ChAT and NMDAR1 in the hippocampus and prefrontal cortex, the brain regions involved with learning and memory function in the present study. Our results demonstrated that the protective effect of various dosages of YCJ on different brain regions is generated via its estrogen-like component. The results derived from the ERα and ERβ immunostaining and counting reflect an increase in the ERα- and ERβ-ir pyramidal neurons in the CA1, CA2, and PF regions. It was observed that there was a significant increase of cell densities in the hippocampus of the sham group. We suggest that this condition may be due to the compounds present in the food pellets. During the last decade, there has been a steady increase in research into the dietary factors that enhance neurogenesis in adults (Poulose et al., 2017). Results of clinical investigations show that polyphenols, vitamins B-9 and E, v-3 PUFAs, and non-nutrient phytochemicals improve adult neurogenesis in adultratsandmice (Shukitt-Hale et al., 2015; Reyes-Izquierdo et al., 2013; Dong et al., 2012; Moriya et al., 2011; Fernández-Fernández et al., 2012).
It has been reported that 58% of YCJ components consists of β-sitosterol alongside different sterols, such as, α spinasterol, stigmasterol, fucosterol, and stigmastatrienol (Rattanaburee et al., 2014) and the β-sitosterol has a similar structure to the animal cholesterol that functions as a sex steroid precursor (Moghadasian, 2000). It has also been reported that coconut extract is rich in phytohormones such as abscisic acid (ABA), auxin, gibberellins (GAs), and other cytokinins (Kobayashi et al., 1997; Ge et al., 2006; Wu and Hu, 2009), and due to its high content of β-sitosterol, stigmasterol and other flavonoids, its methanol extract has an estrogenic effect in rats (Salah et al., 2002). Coconuts contain trans-zeatin, a potent inhibitor of acetylcholinesterase that has been indicated to be effective for the treatment of AD and its associated dysfunctions (Heo et al., 2002; Kim et al., 2008). Besides, trans-zeatin also prevents the formation of amyloid β-protein with an important role in the development and progress of AD (Choi et al., 2009) and showed anti-aging effects on human fibroblast cells (Rattan and Sodagam, 2005). In the current study, the protective role of YCJ against the disturbances of different reactive neurons in the brain of OVX rats is mainly due to its strong estrogenic effect, which facilitates the synthesis of endogenous estrogens.
We verified that the protective effect of various dosages of YCJ on different brain regions is generated via its estrogen-like component by the regression correlation graphs between ChAT-ir and NMDAR1-ir cell numbers with numbers of ER-ir cells. All those p-values are very significant at p-value = 0.000. Moreover, a significant positive correlation was found when correlation analysis was performed. Our results are in agreement with findings derived from other studies and can have important implications in the search by the scientific community for methods to preserve the brain cholinergic system due to the importance of the latter in controlling cerebral blood flow, cortical activity, cognitive functions, and cortical plasticity (Dang, 2010; Hall et al., 2001; Bohacek et al., 2008; Morissette et al., 2008; Schliebs and Arendt, 2006). They also can have important implications in the search for methods to preserve the brain glutamatergic system, which relies on NMDAR1 receptors that participate in the mechanism of long-term potentiation (LTP), the best-understood synaptic model of learning and memory (Sze et al., 2001). However, the double immunohistochemical staining of these ChAT-ir and NMDAR1-ir cells and ER-ir cells will be investigated to confirm this hypothesis in the near future.
Our previous studies demonstrated a significant degree of prevention of AD pathologies in OVX rats treated with YCJ at a daily high dose of 100 mL/kg BW. However, this dosage showed side effects that may be considered unfavorable, such as the deposition of glycogen in the liver. Therefore, in this study, we sought to optimize the YCJ dose by examining the effects of three lower doses, namely 10 mL, 20 mL, and 40 mL/kg BW/day. Our results showed that YCJ at 10 mL/kg BW/day was the optimal neuroprotective dose that preserved cholinergic and glutamatergic neurons in the CA1, CA2, CA3, and PF regions, without affecting the functional parameters of the liver or kidney (please see the details of lipid, liver and renal parameters in Payanglee et al., 2017).
Finally, YCJ seems to act as a selective estrogen receptor modulator (SERM). The results of other parameters investigated in bone, skin, calcium-binding proteins in the GI tract of the same model of both male and female rats confirmed this SERM activities of YCJ (some manuscripts were published, some are being in preparations). Pharmacological interventions using SERMs in estrogen deficiency cases often rely on its binding ability to the estrogen receptors (ERα and ERβ), therefore acting as either agonists or antagonists depending on the pharmacological compounds present as well as the target tissues (Hadji, 2012). Phytoestrogens are plant derivatives that behave differently from estrogen and more like SERMs and have a higher affinity to ERβ (Oseni et al., 2008). We have previously reported that YCJ had beneficial effects on accelerating wound healing in OVX rats, whereby such wounds had a significantly higher degree of expression of ERβ than ERα (Radenahmad et al., 2012). Moreover, our recent study showed that β-sitosterol was a significant component of YCJ, which, in turn, was found to have binding affinities to both ERα and ERβ (Ratanaburee et al.,2014; Dang, 2010). In conclusion, the present study indicates that YCJ may affect preserving cholinergic and glutamatergic neurons via the ChAT and NMDAR1 in the brain regions, at least in part, via a SERM-like activity.
CONCLUSION
The results of this study revealed that: (1) OVX rats showed the reduction of positive neurons due to the deficiency of estrogen hormone; (2) vaginal smear could be used as a preliminary screening estrogenic effect of YCJ; (3) YCJ treatment, at various doses tested, restored the decreased numbers of ChAT-, NMDAR1-, ERα- and ERβ-reactive neurons caused by ovariectomy to normal or close-to-normal levels, and (4) the effects of YCJ were comparable to those of EB treatment. In most cases, the dose of YCJ at 10 ml/kg BW was the best. The higher doses of YCJ (20 ml and 40 ml/kg BW), the more side effects in the liver and kidney and serum analysis parameters. For example, the higher doses of YCJ, the higher the total cholesterol, LDL, and triglyceride levels (please see details in Payanglee et al., 2017). These findings indicate that histological methods can effectively provide scientific evidence of neurodegenerative processes and treatment mechanisms. These findings suggest the potential therapeutic property of YCJ in preventing AD pathology in female rats caused by a lack of estrogen.
ACKNOWLEDGEMENTS
This work was supported by the government budget of the Prince of Songkla University, Grant code: SCI580566S. We thank the late Dr. Brian Hodgson from the Faculty of Pharmaceutical Science, Prince of Songkla University, for assistance with the English correction.
Related articles
 Fig. 1A.- Micrographs of vaginal smears using the Marcondes’s method in all four stages. Proestrus = vaginal smear dominated by nucleated epithelial cells, that occur singly or in sheets; Estrus = period primarily consisting of cornified cells; Metestrus = many leukocytes appearing in this period along with a few cornified cells; Diestrus = smear consisting almost entirely of leukocytes; N = nucleated epithelial cells; L = leukocytes; C = cornified cells. Scale bars = 200 µm.
Fig. 1A.- Micrographs of vaginal smears using the Marcondes’s method in all four stages. Proestrus = vaginal smear dominated by nucleated epithelial cells, that occur singly or in sheets; Estrus = period primarily consisting of cornified cells; Metestrus = many leukocytes appearing in this period along with a few cornified cells; Diestrus = smear consisting almost entirely of leukocytes; N = nucleated epithelial cells; L = leukocytes; C = cornified cells. Scale bars = 200 µm.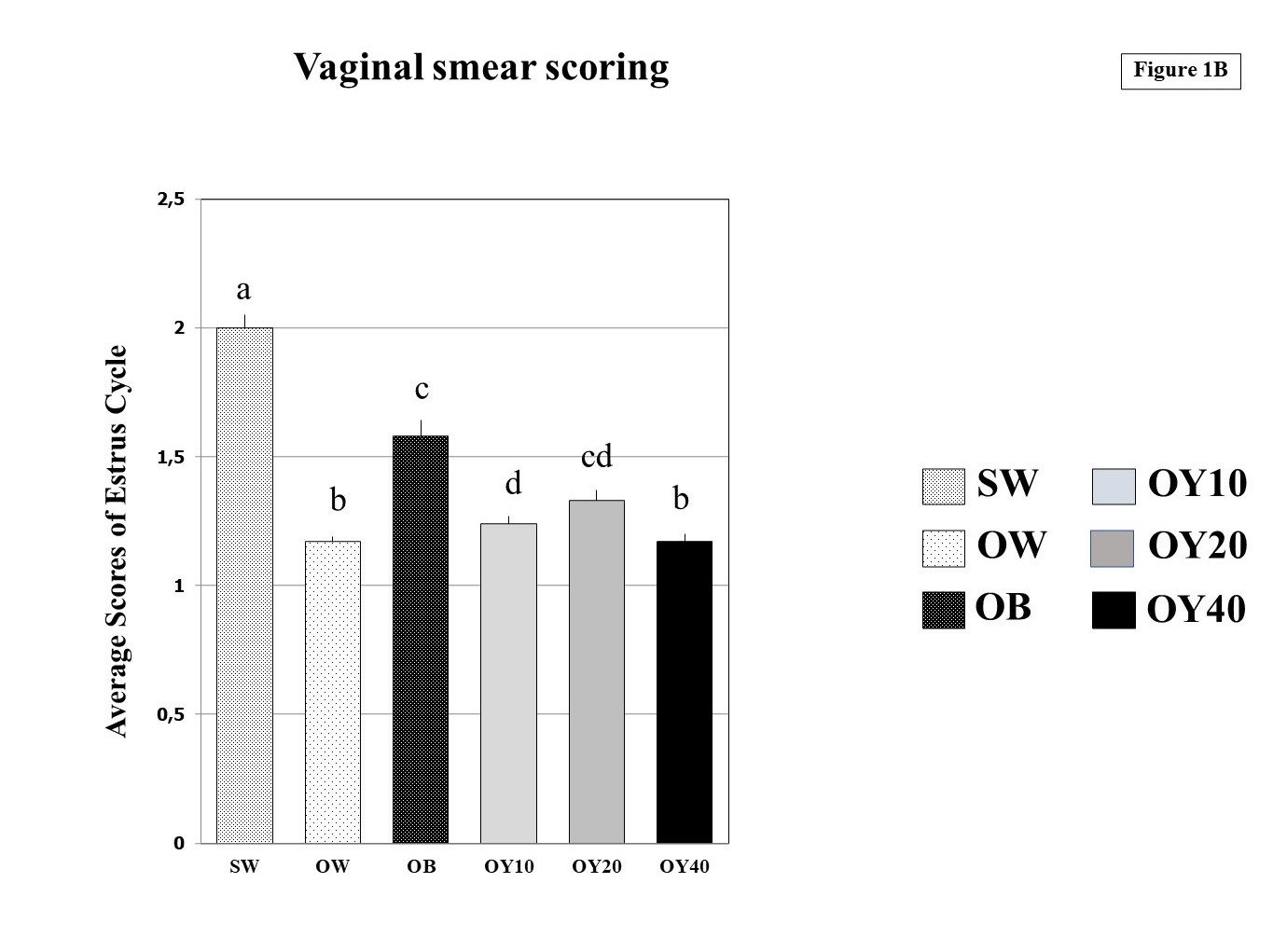 Fig. 1B.- Means of vaginal smear scoring of the estrus cycle for a period of 70 days in the six groups examined. Definition of each group was described in Table 1a. All different superscripts indicate statistical significance at p<0.05 level. SW = sham-operated group; OW = ovariectomized group; OB = ovariectomized rat receiving estradiol benzoate (EB) 2.5 µg/kgBW/day; OY10 = ovariectomized rat receiving YCJ 10 mL/kgBW/day; OY20 = ovariectomized rat receiving YCJ 20 mL/kgBW/day; OY40 = ovariectomized rat receiving YCJ 40 mL/kgBW/day.
Fig. 1B.- Means of vaginal smear scoring of the estrus cycle for a period of 70 days in the six groups examined. Definition of each group was described in Table 1a. All different superscripts indicate statistical significance at p<0.05 level. SW = sham-operated group; OW = ovariectomized group; OB = ovariectomized rat receiving estradiol benzoate (EB) 2.5 µg/kgBW/day; OY10 = ovariectomized rat receiving YCJ 10 mL/kgBW/day; OY20 = ovariectomized rat receiving YCJ 20 mL/kgBW/day; OY40 = ovariectomized rat receiving YCJ 40 mL/kgBW/day. Fig. 2.- Serum estradiol (E2) levels (pg/mL) of the 7 groups examined. All different superscripts indicate statistical significance at p<0.05 level. BL = baseline control group; SW = sham-operated group; OW = ovariectomized group; OB = ovariectomized rat receiving estradiol benzoate (EB) 2.5 µg/kgBW/day; OY10 = ovariectomized rat receiving YCJ 10 mL/kgBW/day; OY20 = ovariectomized rat receiving YCJ 20 mL/kgBW/day; OY40 = ovariectomized rat receiving YCJ 40 mL/kgBW/day.
Fig. 2.- Serum estradiol (E2) levels (pg/mL) of the 7 groups examined. All different superscripts indicate statistical significance at p<0.05 level. BL = baseline control group; SW = sham-operated group; OW = ovariectomized group; OB = ovariectomized rat receiving estradiol benzoate (EB) 2.5 µg/kgBW/day; OY10 = ovariectomized rat receiving YCJ 10 mL/kgBW/day; OY20 = ovariectomized rat receiving YCJ 20 mL/kgBW/day; OY40 = ovariectomized rat receiving YCJ 40 mL/kgBW/day.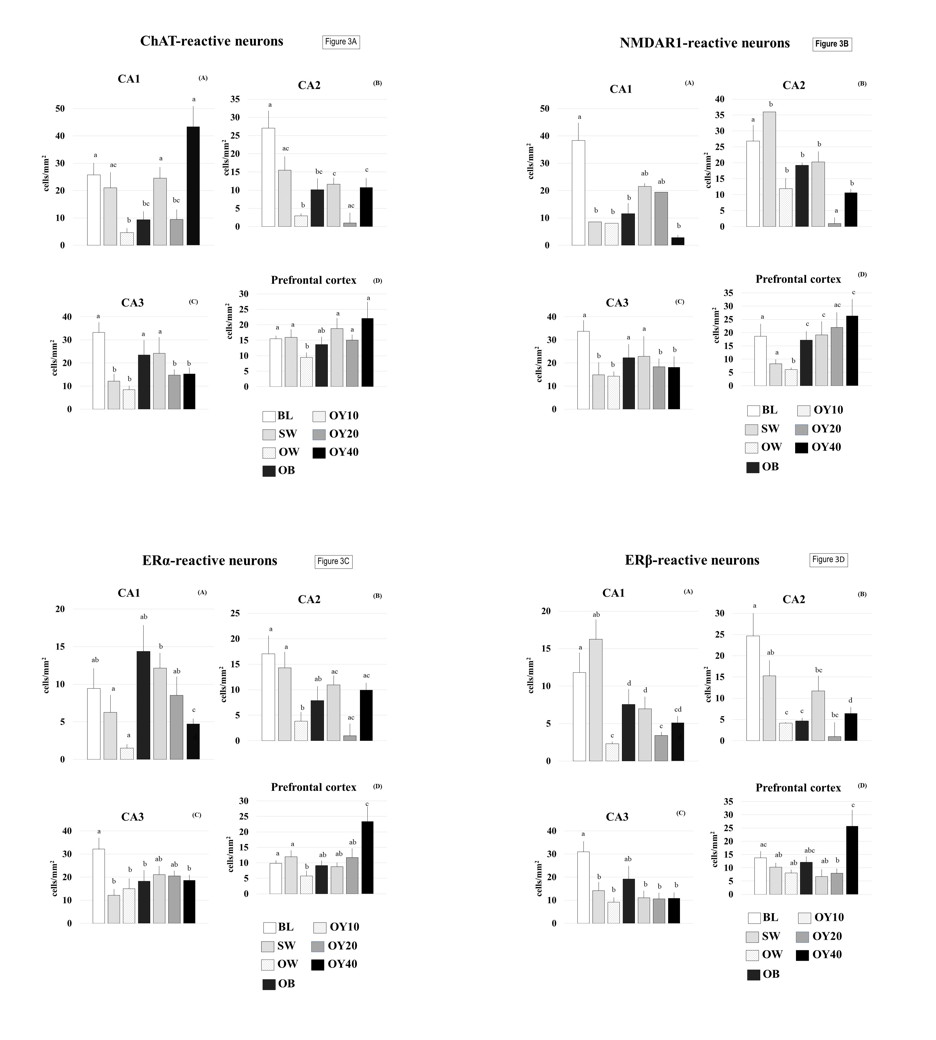 Fig. 3.- Number of ChAT-reactive neurons (3A), NMDAR1 (3B), ERα (3C), ERβ (3D) of the cornu ammonis (CA) of the hippocampus and areas of cerebral cortices in all groups of the rats tested. All different superscripts indicate statistical significance at p<0.05 level. BL = baseline control group; SW = sham-operated group; OW = ovariectomized group; OB = ovariectomized rat receiving estradiol benzoate (EB) 2.5 µg/kgBW/day; OY10 = ovariectomized rat receiving YCJ 10 mL/kgBW/day; OY20 = ovariectomized rat receiving YCJ 20 mL/kgBW/day; OY40 = ovariectomized rat receiving YCJ 40 mL/kgBW/day.
Fig. 3.- Number of ChAT-reactive neurons (3A), NMDAR1 (3B), ERα (3C), ERβ (3D) of the cornu ammonis (CA) of the hippocampus and areas of cerebral cortices in all groups of the rats tested. All different superscripts indicate statistical significance at p<0.05 level. BL = baseline control group; SW = sham-operated group; OW = ovariectomized group; OB = ovariectomized rat receiving estradiol benzoate (EB) 2.5 µg/kgBW/day; OY10 = ovariectomized rat receiving YCJ 10 mL/kgBW/day; OY20 = ovariectomized rat receiving YCJ 20 mL/kgBW/day; OY40 = ovariectomized rat receiving YCJ 40 mL/kgBW/day.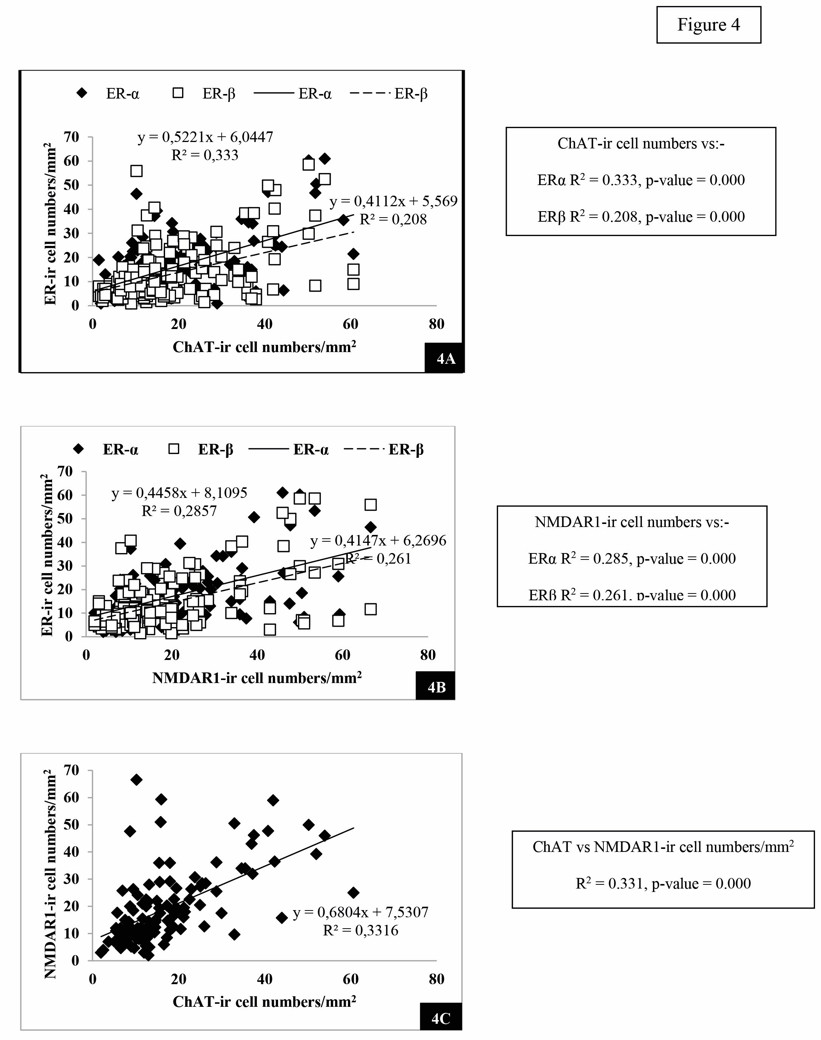 Fig. 4.- Plot of number of the ChAT-reactive neurons against the number of ERα- and ERβ- reactive neurons (4A), the NMDAR1-reactive neurons against the number of ERα- and ERβ- reactive neurons (4B) and the ChAT-reactive neurons against the number of NMDAR1-reactive neurons (4C), from the same rats and from all animal groups.
Fig. 4.- Plot of number of the ChAT-reactive neurons against the number of ERα- and ERβ- reactive neurons (4A), the NMDAR1-reactive neurons against the number of ERα- and ERβ- reactive neurons (4B) and the ChAT-reactive neurons against the number of NMDAR1-reactive neurons (4C), from the same rats and from all animal groups. Fig. 5.- Examples of reactive neurons of YCJ-treated groups: (A) ChAT-reactive neurons in the CA2, (B)NMDAR1-reactive neurons in the CA1, (C) ERα- reactive neurons in the CA1 of the hippocampus and, (D) ERβ-reactive neurons in the prefrontal cortex of the brains. Scale bars = 50 µm.
Fig. 5.- Examples of reactive neurons of YCJ-treated groups: (A) ChAT-reactive neurons in the CA2, (B)NMDAR1-reactive neurons in the CA1, (C) ERα- reactive neurons in the CA1 of the hippocampus and, (D) ERβ-reactive neurons in the prefrontal cortex of the brains. Scale bars = 50 µm.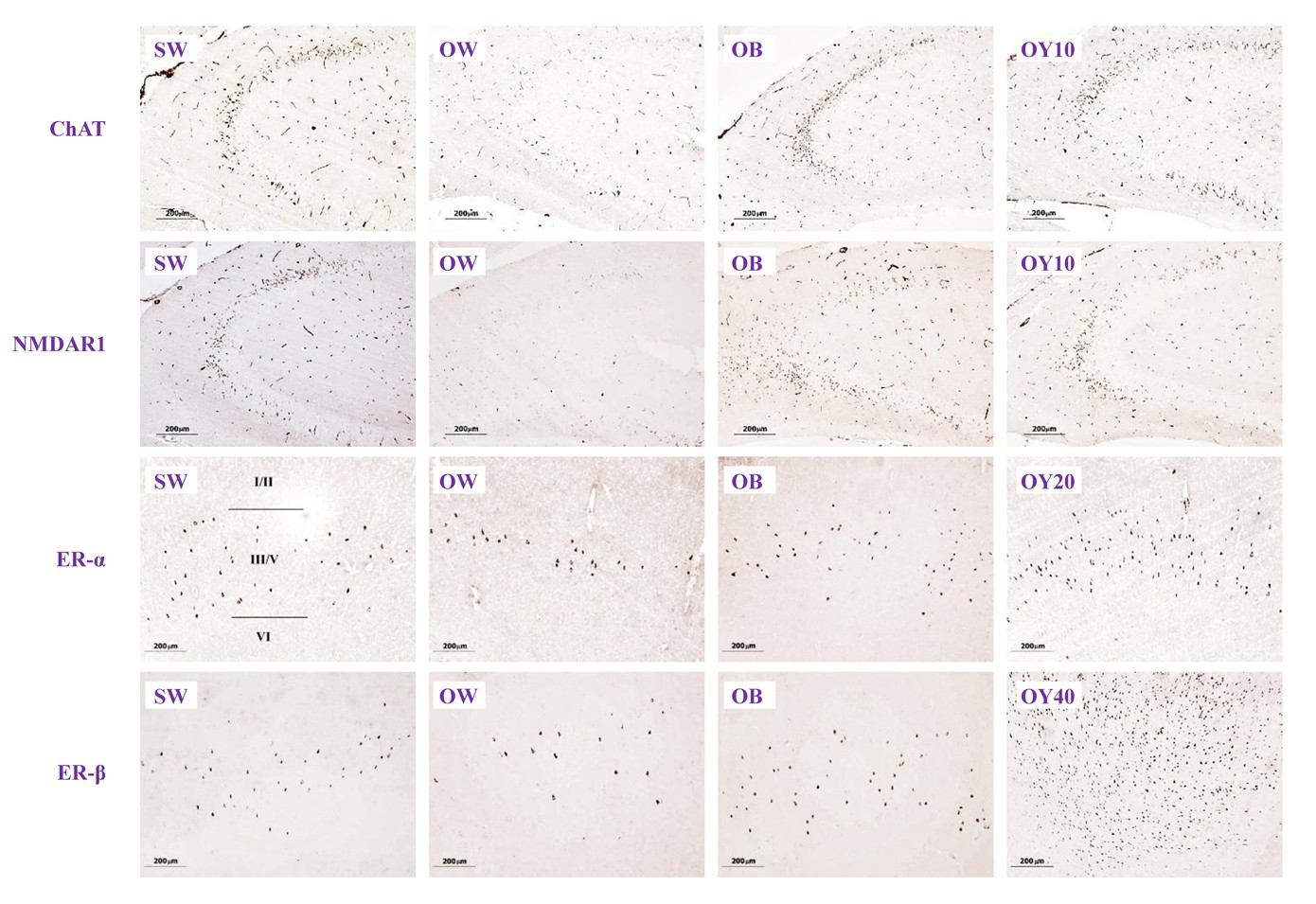 Fig. 6A.- Immunohistochemical sections showing the degree expression of ChAT-, NMDAR1-, ERα- and ERβ- positive neurons in the hippocampus (HP) and prefrontal cortex (PF) brain sections (10x). SW = sham-operated group; OW = ovariectomized group; OB = ovariectomized rat receiving estradiol benzoate (EB) 2.5 μg/kgBW/day; OY10 = ovariectomized rat receiving YCJ 10 mL/kgBW/day; OY20 = ovariectomized rat receiving YCJ 20 mL/kgBW/day; OY40 = ovariectomized rat receiving YCJ 40 mL/kgBW/day..
Fig. 6A.- Immunohistochemical sections showing the degree expression of ChAT-, NMDAR1-, ERα- and ERβ- positive neurons in the hippocampus (HP) and prefrontal cortex (PF) brain sections (10x). SW = sham-operated group; OW = ovariectomized group; OB = ovariectomized rat receiving estradiol benzoate (EB) 2.5 μg/kgBW/day; OY10 = ovariectomized rat receiving YCJ 10 mL/kgBW/day; OY20 = ovariectomized rat receiving YCJ 20 mL/kgBW/day; OY40 = ovariectomized rat receiving YCJ 40 mL/kgBW/day..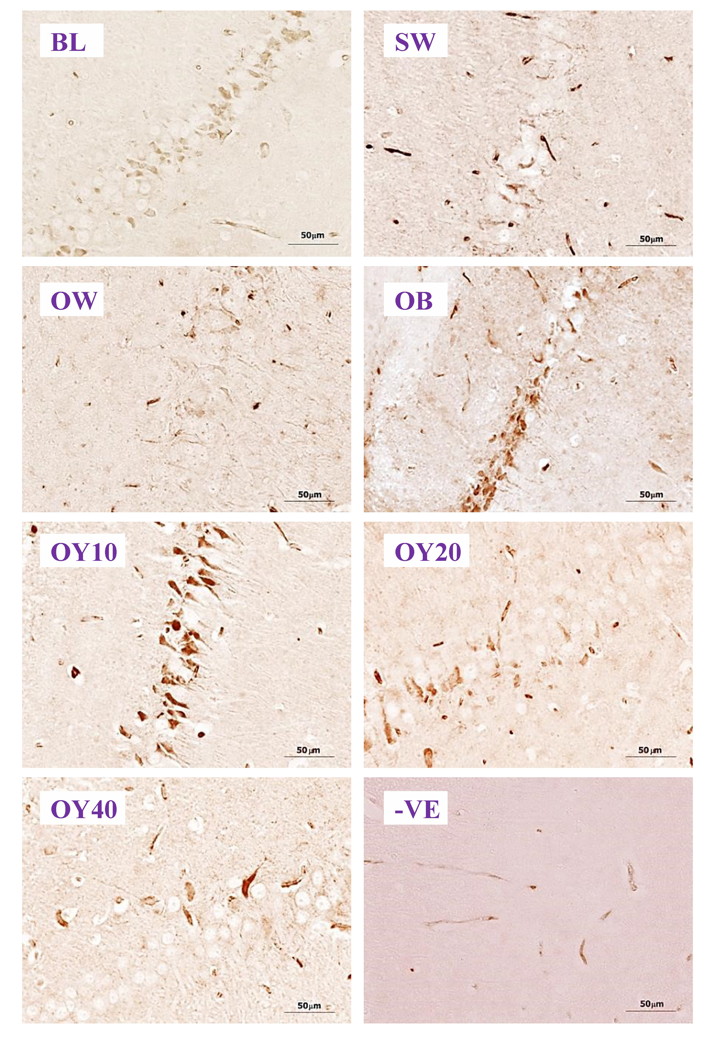 Fig. 6B.- Examples of higher magnification (40x) of ChAT-ir positive neurons in the CA1 of hippocampus. BL= baseline group; SW = sham-operated group; OW = ovariectomized group; OB = ovariectomized rat receiving estradiol benzoate (EB) 2.5 μg/kgBW/day; OY10 = ovariectomized rat receiving YCJ 10 mL/kgBW/day; OY20 = ovariectomized rat receiving YCJ 20 mL/kgBW/day; OY40 = ovariectomized rat receiving YCJ 40 mL/kgBW/day; -VE = negative control group.
Fig. 6B.- Examples of higher magnification (40x) of ChAT-ir positive neurons in the CA1 of hippocampus. BL= baseline group; SW = sham-operated group; OW = ovariectomized group; OB = ovariectomized rat receiving estradiol benzoate (EB) 2.5 μg/kgBW/day; OY10 = ovariectomized rat receiving YCJ 10 mL/kgBW/day; OY20 = ovariectomized rat receiving YCJ 20 mL/kgBW/day; OY40 = ovariectomized rat receiving YCJ 40 mL/kgBW/day; -VE = negative control group.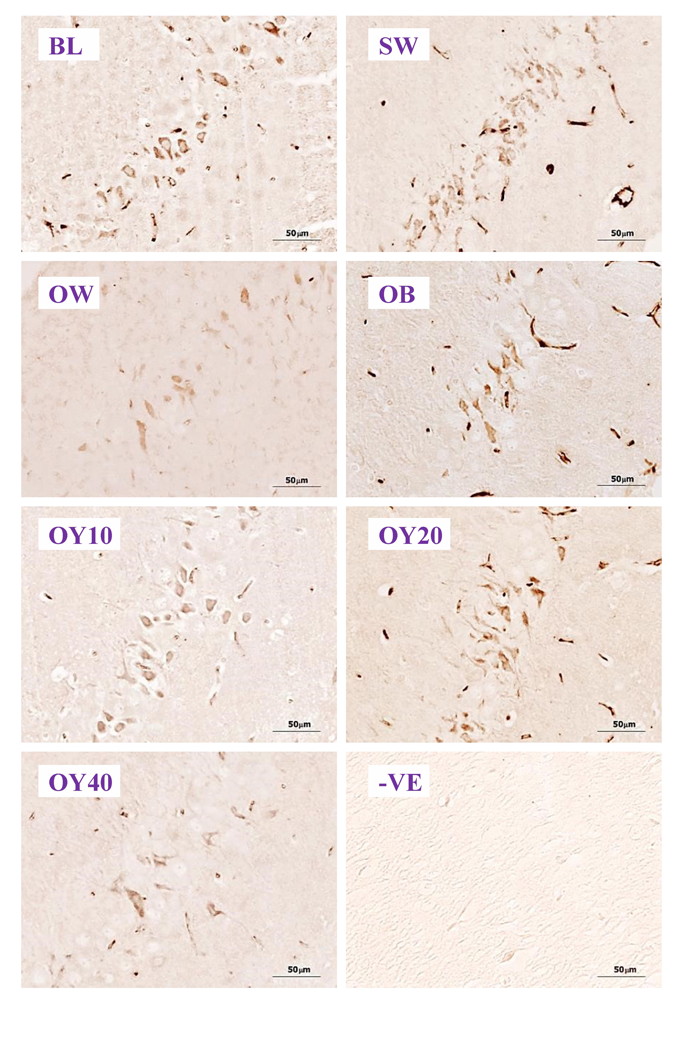 Fig. 6C.- Examples of higher magnification (40x) of NMDAR1-ir positive neurons in the CA2 of the hippocampus. BL= baseline group; SW = sham-operated group; OW = ovariectomized group; OB = ovariectomized rat receiving estradiol benzoate (EB) 2.5 μg/kgBW/day; OY10 = ovariectomized rat receiving YCJ 10 mL/kgBW/day; OY20 = ovariectomized rat receiving YCJ 20 mL/kgBW/day; OY40 = ovariectomized rat receiving YCJ 40 mL/kgBW/day; -VE = negative control group.
Fig. 6C.- Examples of higher magnification (40x) of NMDAR1-ir positive neurons in the CA2 of the hippocampus. BL= baseline group; SW = sham-operated group; OW = ovariectomized group; OB = ovariectomized rat receiving estradiol benzoate (EB) 2.5 μg/kgBW/day; OY10 = ovariectomized rat receiving YCJ 10 mL/kgBW/day; OY20 = ovariectomized rat receiving YCJ 20 mL/kgBW/day; OY40 = ovariectomized rat receiving YCJ 40 mL/kgBW/day; -VE = negative control group.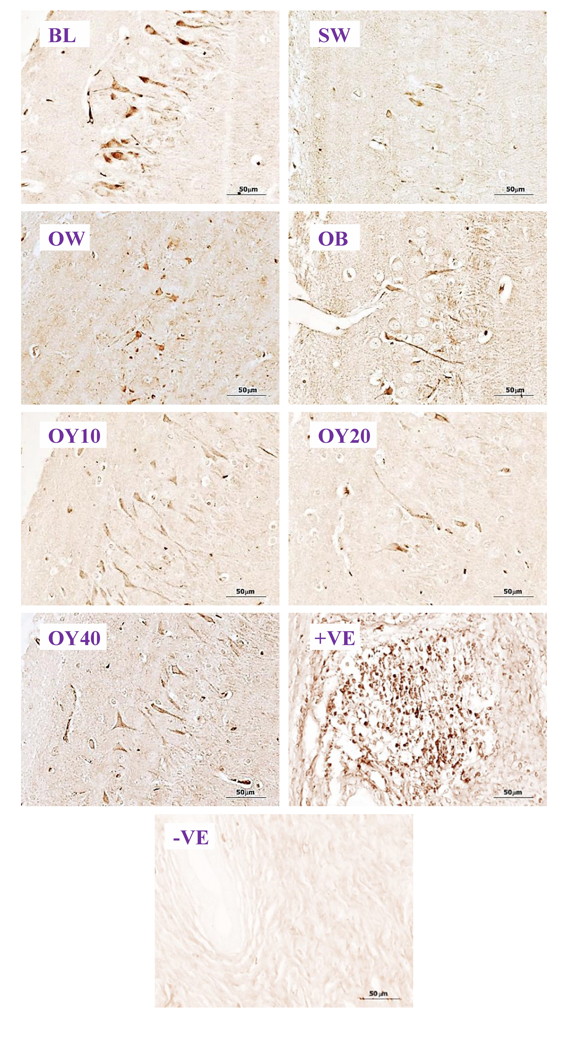 Fig. 6D.- Examples of higher magnification (40x) of ERα-ir positive neurons in the CA3 of hippocampus. BL= baseline group; SW = sham-operated group; OW = ovariectomized group; OB = ovariectomized rat receiving estradiol benzoate (EB) 2.5 μg/kgBW/day; OY10 = ovariectomized rat receiving YCJ 10 mL/kgBW/day; OY20 = ovariectomized rat receiving YCJ 20 mL/kgBW/day; OY40 = ovariectomized rat receiving YCJ 40 mL/kgBW/day; +VE = positive control group (uterus for ERα and ovary for ERβ); -VE = negative control group.
Fig. 6D.- Examples of higher magnification (40x) of ERα-ir positive neurons in the CA3 of hippocampus. BL= baseline group; SW = sham-operated group; OW = ovariectomized group; OB = ovariectomized rat receiving estradiol benzoate (EB) 2.5 μg/kgBW/day; OY10 = ovariectomized rat receiving YCJ 10 mL/kgBW/day; OY20 = ovariectomized rat receiving YCJ 20 mL/kgBW/day; OY40 = ovariectomized rat receiving YCJ 40 mL/kgBW/day; +VE = positive control group (uterus for ERα and ovary for ERβ); -VE = negative control group.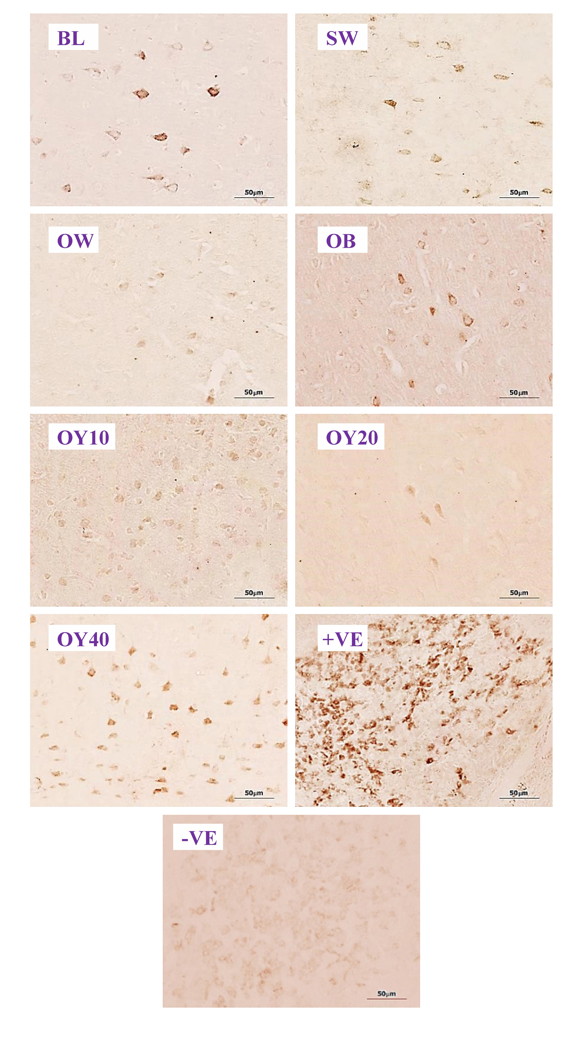 Fig. 6E.- Examples of higher magnification (40x) of ERβ-ir positive neurons in the prefrontal cortex. BL= baseline group; SW = sham-operated group; OW = ovariectomized group; OB= ovariectomized rat receiving estradiol benzoate (EB) 2.5 μg/kgBW/day; OY10 = ovariectomized rat receiving YCJ 10 mL/kgBW/day; OY20 = ovariectomized rat receiving YCJ 20 mL/kgBW/day; OY40 = ovariectomized rat receiving YCJ 40 mL/kgBW/day; +VE = positive control group (uterus for ERα and ovary for ERβ); -VE = negative control group.
Fig. 6E.- Examples of higher magnification (40x) of ERβ-ir positive neurons in the prefrontal cortex. BL= baseline group; SW = sham-operated group; OW = ovariectomized group; OB= ovariectomized rat receiving estradiol benzoate (EB) 2.5 μg/kgBW/day; OY10 = ovariectomized rat receiving YCJ 10 mL/kgBW/day; OY20 = ovariectomized rat receiving YCJ 20 mL/kgBW/day; OY40 = ovariectomized rat receiving YCJ 40 mL/kgBW/day; +VE = positive control group (uterus for ERα and ovary for ERβ); -VE = negative control group.BITZER J, KENEMANS P, MUECK AO (2008) Breast cancer risk in postmenopausal women using testosterone in combination with hormone replacement therapy. Maturitas, 59(3): 209-218.
BOHACEK J, BEARL AM, DANIEL JM (2008) Long‐term ovarian hormone deprivation alters the ability of subsequent oestradiol replacement to regulate choline acetyltransferase protein levels in the hippocampus and prefrontal cortex of middle-aged rats. J Neuroendocrinol, 20(8): 1023-1027.
BURTON JL, WELLS M (2002) The effect of phytoestrogens on the female genital tract. J Clin Pathol, 55(6): 401-407.
CARROLL JC, ROSARIO ER, CHANG L, STANCZYK FZ, ODDO S, LAFERLA FM, PIKE CJ (2007) Progesterone and estrogen regulate Alzheimer-like neuropathology in female 3xTg-AD mice. J Neurosci, 27(48): 13357-13365.
COLLINGRIDGE GL, SINGER W (1990) Excitatory amino acid receptors and synaptic plasticity. Trends Pharmacol Sci, 11(7): 290-296.
CHOI SJ, JEONG CH, CHOI SG, CHUN JY, KIM YJ, LEE JM, SHIN DH, HEO HJ (2009) Zeatin prevents amyloid beta-induced neurotoxicity and scopolamine-induced cognitive deficits. J Med Food,12: 271-277.
DANG Z (2010) Comparison of relative binding affinities to fish and mammalian estrogen receptors: the regulatory implications. Toxicol Lett, 192(3): 298-315.
DONG S, ZENG Q, MITCHELL ES, XIU J, DUAN Y, LI C, TIWARI JK, HU Y, CAO X, ZHAO Z (2012) Curcumin enhances neurogenesis and cognition in aged rats: implications for transcriptional interactions related to growth and synaptic plasticity. PlosOne, 7(2): e31211.
FERNÁNDEZ-FERNÁNDEZ L, COMES G, BOLEA I, VALENTE T, RUIZ J, MURTRA P, RAMIREZ B, ANGLES N, REGUANT J, MORELLO JR, BOADA M, HIDALGO J, ESCORIHUELA M, UNZETA M (2012) LMN diet, rich in polyphenols and polyunsaturated fatty acids, improves mouse cognitive decline associated with aging and Alzheimer’s disease. Behav Brain Res, 228(2): 261-271.
FERNÁNDEZ-TOMÉ P, BRERA B, ARÉVALO MA, DE CEBALLOS ML (2004) β-amyloid 25-35 inhibits glutamate uptake in cultured neurons and astrocytes: modulation of uptake as a survival mechanism. Neurobiology Dis, 15(3): 580-589.
FERREIRA-VIEIRA TH, GUIMARAES IM, SILVA FR, RIBEIRO FM (2016) Alzheimer’s disease: Targeting the cholinergic system. Curr Neuropharmacol, 14(1): 101-115.
GE L, TAN S, YONG JWH, TAN SN (2006) Capillary electrophoresis for cytokinin analyses: A review. Electrophoresis, 27: 4779-4791.
GREENAMYRE JT (1986) The role of glutamate in neurotransmission and in neurologic disease. Arch Neurol, 43(10): 1058-1063.
HADJI P (2012) The evolution of selective estrogen receptor modulators in osteoporosis therapy. Climacteric, 15(6): 513-523.
HALL JM, COUSE JF, KORACH KS (2001) The multifaceted mechanisms of estradiol and estrogen receptor signaling. J Biol Chem, 276(40): 36869-36872.
HEO HJ, HONG SC, CHO HY, HONG B, KIM HK, KIM EK, SHIN DH (2002) Inhibitory effect of zeatin, isolated from Fiatouavillosa, on acetylcholinesterase activity from PC12 cells. Mol Cells, 13: 113-117.
JAMSHED N, OZAIR FF, AGGAWAL P, EKKA M (2014) Alzheimer disease in postmenopausal women: Intervene in the critical window period. J Mid-life Health, 5(1): 38-40.
JIN YK, BAE HS, LEE JY, YUM SY, KIM KM, KOO OJ, AILIA MJ (2018) The effect of gonadotropin-releasing hormone agonist on superovulation and estrous synchronization in female Sprague Dawley rat. Reprod Fert Develop, 30(1): 239-239.
KOBAYASHI H, MORISAKI N, TAGO Y, HASHIMOTO Y, IWASAKI S, KAWACHI E, NAGATA R, SHUDO K (1997) Structural identification of a major cytokinin in coconut milk as14-O-(3-O-[β-Dgalactopyranosyl-(1-->2)-α-D-galactopyranosyl-(1-->3)-α-L-arabinofuranosyl]-4-O-(α-L-arabinofuranosyl)-β-D-galactopyranosyl)-trans-zeatinriboside. Chem Pharm Bull, 45: 260-264.
KIM MJ, CHOI SJ, LIM ST, KIM HK, KIM YJ, YOON HG, SHIN DH (2008) Zeatin supplement improves scopolamine-induced memory impairment in mice. Biosci Biotechnol Biochem, 72: 577-581.
LAUDERBACK CM, HACKETT JM, HUANG FF, KELLER JN, SZWEDA LI, MARKESBERY WR, BUTTERFIELD DA (2001) The glial glutamate transporter, GLT-1, is oxidatively modified by 4-hydroxy-2-nonenal in the Alzheimer’s disease brain: the role of Aβ1–42. J Neurochem, 78(2): 413-416.
LAWS SC, CAREY SA, FERRELL JM, BODMAN GJ, COOPER RL (2000) Estrogenic activity of octylphenol, nonylphenol, bisphenol A and methoxychlor in rats. Toxicol Sci, 54(1): 154-167.
MARAGOS WF, GREENAMYRE JT, PENNEY JR JB, YOUNG AB (1987) Glutamate dysfunction in Alzheimer’s disease: a hypothesis. Trends Neurosci, 10(2): 65-68.
MARCONDES FK, BIANCHI FJ, TANNO AP (2002) Determination of the estrous cycle phases of rats: some helpful considerations. Braz J Biol, 62(4A): 609-614.
MASTERS CL, BATEMAN R, BLENNOW K, ROWE CC, SPERLING RA, CUMMINGS JL (2015) Alzheimer’s disease. Nat Rev Dis Primer, 1: 15056.
MCCULLOUGH ML, PATEL AV, PATEL R, RODRIGUEZ C, FEIGELSON HS, BANDERA EV, GANSLER T, THUN MJ, CALLE EE (2008) Body mass and endometrial cancer risk by hormone replacement therapy and cancer subtype. Cancer Epidem Biomar, 17(1): 73-79.
MOGHADASIAN MH (2000) Pharmacological properties of plant sterols in vivo and in vitro observations. Life Sci, 67(6): 605-615.
MORISSETTE M, LE SAUX M, D’ASTOUS M, JOURDAIN S, AL SWEIDI S, MORIN N, ESTRADA-CAMARENA E, MENDEZ P, GARCIA-SEGURA LM, DI PAOLO T (2008) Contribution of estrogen receptors alpha and beta to the effects of estradiol in the brain. J Steroid Biochem, 108(3-5): 327-338.
MORIYA J, CHEN R, YAMAKAWA JI, SASAKI, K, ISHIGAKI Y, TAKAHASHI T (2011) Resveratrol improves hippocampal atrophy in chronic fatigue mice by enhancing neurogenesis and inhibiting apoptosis of granular cells. Biol Pharm Bull, 34(3): 354-359.
OSENI T, PATEL R, PYLE J, JORDAN VC (2008) Selective estrogen receptor modulators and phytoestrogens. Planta Med, 74(13): 1656-1665.
PALMER AM, GERSHON S (1990) Is the neuronal basis of Alzheimer’s disease cholinergic or glutamatergic? FASEB J, 4(10): 2745-2752.
PARHIZKAR S, LATIFFAH A, LATIFF LA, RAHMAN SA (2011) Assessing estrogenic activity of Nigella sativa in ovariectomized rats using vaginal cornification assay. Afr J Pharm Pharmaco, 5(2): 137-142.
PAYANGLEE K, CHONPATHOMPIKUNLERT P, PANITYAKUL T, RADENAHMAD N (2017) Beneficial effects of young coconut juice on preserving neuronal cell density, lipid, renal, and liver profiles in ovariectomizedrats: A preliminary study. Songklanakarin J Sci Technol, 39(2): 237-243.
PLASSMAN BL, LANGA KM, FISHER GG, HEERINGA SG, WEIR DR, OFSTEDAL MB, BURKE JR, HURD MD, POTTER GG, RODGERS WL, STFFENS DC, WILLIS R, WALLACE RB (2007) Prevalence of dementia in the United States: The aging, demographics, and memory study. Neuroepidemiology, 29: 125-132.
POULOSE SM, MILLER MG, SCOTT T, SHUKITT-HALE B (2017) Nutritional factors affecting adult neurogenesis and cognitive function. Adv Nutr, 8(6): 804-811.
RADENAHMAD N, VONGVATCHARANON U, WITHYACHUMNARNKUL B, CONNOR RJ (2006) Serum levels of 17β-estradiol in ovariectomized rats fed young-coconut-juice and its effect on wound healing. Songklanakarin J Sci Technol, 28: 897-910.
RADENAHMAD N, SALEH F, SAWANGJAROEN K, RUNDORN W, WITHYACHUMNARNKUL B, CONNOR JR (2009) Young coconut juice significantly reduces histopathological changes in the brain that is induced by hormonal imbalance: A possible implication to postmenopausal women. Histol Histopathol, 24(6): 667-674.
RADENAHMAD N, SALEH F, SAWANGJAROEN K, VONGVATCHARANON U, SUBHADHIRASAKUL P, RUNDORN W, WITHYACHUMNARNKUL B, CONNOR JR (2011) Young coconut juice, a potential therapeutic agent that could significantly reduce some pathologies associated with Alzheimer’s disease: novel findings. British J Nutr, 105(5): 738-746.
RADENAHMAD N, SALEH F, SAYOH I, SAWANGJAROEN K, SUBHADHIRASAKUL P, BOONYOUNG P, RUNDORN W, MITRANUN W (2012) Young coconut juice can accelerate the healing process of cutaneous wounds. BMC Complem Altern M, 12(1): 252.
RADENAHMAD N, BOONYOUNG P, KAMKAEW K, CHANCHULA K, KIRIRAT P (2014) Effects of young coconut juice on the numbers of argyrophil endocrine cells in the gastrointestinal tract of male rats: Novel preliminary findings. Songklanakarin J Sci Technol, 36: 599-606.
RADENAHMAD N, SUWANSA-ARD S, SAYOH I (2015) Young coconut juice accelerates cutaneous wound healing by downregulating macrophage migration inhibitory factor (MIF) in ovariectomized rats: Preliminary novel findings. Songklanakarin J Sci Technol, 37: 417-423.
RATTAN SIS, SODAGAM L (2005) Gerontomodulatory and youth-preserving effects of zeatin on human skin fibroblasts undergoing aging in vitro. Rejuv Res, 8: 46-57.
RATTANABUREE P, AMNUAIKIT T, RADENAHMAD N, PURIPATTANAVONG J (2014) Phytochemical study and its quantity and quality of fresh and freeze-dried young coconut juice (Cocosnucifera L.). In: Advanced Materials Research, 884: 490-493). Trans Tech Ltd, Publications, Switzerland.
REYES-IZQUIERDO T, NEMZER B, SHU C, HUYNH L, ARGUMEDO R, KELLER R, PIETRZKOWSKI Z (2013) Modulatory effect of coffee fruit extract on plasma levels of brain-derived neurotrophic factor in healthy subjects. British J Nutr, 110(3): 420-425.
SALAH AM, GATHUMBI J, VIERLING W, WAGNER H (2002) Estrogenic and cholinergic properties of the methanol extract of Ruelliapraetermissa Sceinf. ex. Lindau (Acanthaceae) in female rats. Phytomed, 9: 52-55.
SCHLIEBS R, ARENDT T (2006) The significance of the cholinergic system in the brain during aging and in Alzheimer’s disease. J Neural Transm, 113(11): 1625-1644.
SEZGIN Z, DINCER Y (2014) Alzheimer’s disease and epigenetic diet. Neurochem Int, 78: 105-116.
SHUKITT-HALE B, BIELINSKI DF, LAU FC, WILLIS LM, CAREY AN, JOSEPH JA (2015) The beneficial effects of berries on cognition, motor behavior and neuronal function in aging. British J Nutr, 114(10): 1542-1549.
SUWANPAL P, RADENAHMAD N, YUSUH M, EKSOMTRAMATE M, RUANGSRI P, CHANTANASUKSILPA A (2011) Effects of young-coconut juice on increasing mandibular cancellous bone in orchidectomized rats: Preliminary novel findings. Songklanakarin J Sci Technol, 33(6): 617-623.
SZE CI, BI H, KLEINSCHMIDT-DEMASTERS BK, FILLEY CM, MARTIN LJ (2001) N-methyl-D-aspartate receptor subunit proteins and their phosphorylation status are altered selectively in Alzheimer’s disease. J Neurol Sci, 182(2): 151-159.
SZEGŐ ÉM, CSORBA A, JANÁKY T, KÉKESI KA, ÁBRAHÁM IM, MÓROTZ GM, PENKE B, PALKOVITS M, MURVAI U, KELLERMAYER MSZ, KARDOS J (2011) Effects of estrogen on beta-amyloid-induced cholinergic cell death in the nucleus basalismagnocellularis. Neuroendocrinology, 93(2): 90-105.
VAN DIJK GM, KAVOUSI M, TROUP J, FRANCO OH (2015) Health issues for menopausal women: The top 11 conditions have common solutions. Maturitas, 80(1): 24-30.
WU Y, HU B (2009) Simultaneous determination of several phytohormones in natural coconut juice by hollow fiber-based liquid-liquid-liquid microextraction-high performance liquid chromatography. J Chromatogr, 1216: 7657-7663.
YUSUH M, PHOCHANUKOON N, RADENAHMAD N, EKSOMTRAMATE M, RUANGSRI P, CHANTANASUKSILPA A, NITIRUANGJARAS A (2010) Changes of condyle cartilage in orchidectomized rats fed with young coconut juice: Novel preliminary findings. Songklanakarin J Sci Technol, 32(4): 333-339.
ZHOU B, SUN Q, CONG R, GU H, TANG N, YANG L, WANG B (2008) Hormone replacement therapy and ovarian cancer risk: a meta-analysis. GynecolOncol, 108(3): 641-651.