It is imperative to have a lot of discretion regarding animals’ use in research and teaching activities. Consequently, the search for alternative methods that do not cause academic or scientific damage is essential. This research aims to determine the maximum rupture force and the rupture elongation of the skin and the students’ evaluation of the embalmed dogs’ cadaver for veterinary surgery classes. Cadavers were injected with 120 mL/kg of a 20% sodium chloride, 1% nitrite and 1% sodium nitrate solution, and 150 mL/kg of alcohol with 5% glycerin and kept in vacuum packages between 0 to 4°C. Eight dogs constituted group 1, and three skin samples were collected on day 0 (fresh samples/before fixation) and during the next seven consecutive days. Only days 2 and 6 were different from the control. Group 2 was analyzed by 46 undergraduate students during the veterinary surgery classes, who completed a form about malleability and incision/suture of the tissue. Using a scale from zero to ten, the reached value was 7.95, and 100% of the students approved the use of embalmed dogs for surgical training. The anatomical technique had an excellent cost-benefit ratio in addition to reduced environmental impact. The method maintained malleability and quality of incision and suture in surgical practice.
Vacuum packaged embalmed dogs for veterinary surgery practicing
Isabela Del Ponti1, Giovana C. Vieira1, Laura G. Soares1, Alessandra Rodrigues1, Natália T.B. Costa1, Geovana C. Ferreira1, Alisson D.S. Fechis2, Andréa B.P.S. Queiroz1, Fabrício S. Oliveira1
1 Department of Animal Morphology and Physiology, School of Agrarian and Veterinary Science, São Paulo State University (UNESP), Jaboticabal, São Paulo, Brazil
2 Department of Veterinary Animal Pathobiology, School of Agrarian and Veterinary Sciences, São Paulo State University (UNESP), Jaboticabal, São Paulo, Bra
SUMMARY
Sign up or Login
INTRODUCTION
Great wisdom is very important in the use of animals in research and teaching activities. It is therefore important to look for alternative methods that do not bring academic or scientific harm.
Several alternative methods currently optimize animal welfare in the teaching of veterinary surgery (Oliveira, 2008). These methods aim to replace live animals’ use, generating similar or superior learning to the students (Silva et al., 2007). A negative emotional state can hinder more complex cognitive mechanisms, that is, block significant learning. Some students feel uncomfortable and even shocked in classes with live animals, and what happens is just visual memorization and not meaningful learning in many of these situations (Paixão, 2008).
Several kinds of research have demonstrated the effectiveness of using chemically prepared cadavers for veterinary surgery classes. That provides greater acceptance by students and better learning (Silva et al., 2004), and animals that died in shelters, veterinary clinics, and hospitals could be used as substitutes for live animals (Silva et al., 2007; Mathews et al., 2010).
Still, there were no significant differences in surgical performance in veterinary medicine students trained on a cadaver or live animals (Carpenter et al., 1991; Silva et al., 2007). The use of chemically preserved cadavers was approved by 95.7% of the veterinary medicine students (Silva et al., 2007).
Interest in the biomechanical properties of animal biological tissues is increasing. A significant focus is on comparative studies between preserved and fresh samples, generating data that contribute to improved surgical techniques. Besides, the search for alternative materials is a new option for animal experimentation models (Camargo et al., 2014).
The anatomical specimens are fixed to avoid tissue deterioration. Fixation is essential, as it keeps the tissues firm, insoluble, and protected (Rodrigues, 2010). Thus, the excellent conservation does not allow the material to deteriorate and prevents the proliferation of pathogens that may cause disease in laboratory workers (Corrêa, 2003). The most common substances used for cadaver preservation are formaldehyde, glycerin, ethyl alcohol, and phenol (Rodrigues, 2010).
Formaldehyde is the most widely used fixative, commonly in a 10% aqueous solution. It quickly penetrates tissues (six millimeters in twelve hours) and is commonly used in anatomy laboratories (Rodrigues, 2010). In addition to being harmful to health, it poses a severe environmental risk, as the improper handling and disposal of carcasses and effluents can contaminate the environment (WHO, 1991).
The current model that comes closest to reality is the fresh corpse. However, it needs freezing, has a limited working time due to rapid putrefaction, and can be an infection risk. The saturated salt solutions are a simple method, with a low risk of infection and cost, contributing to the wide use of preserved cadavers for surgical training (Hayashi et al., 2016).
The curing salt solution is an alternative to formaldehyde for the long-term preservation of dogs’ anatomical specimens subjected to dissection at the Szent István University of Science, Hungary (Werdelmann and Gerics, 2016) and Berlin (Janczyk et al., 2010). The cadavers presented high quality of softness and color of the tissues immersed in the solution, contrasting with the stiffness and gray color caused by formaldehyde (Werdelmann and Gerics, 2016), without environmental and health risks (Janczyk et al., 2010).
Surgical training was performed on dogs’ cadavers fixed with ethyl alcohol (AE) and preserved in 30% sodium chloride solution (30% SCAS) for eight months with good biomechanical quality of tissues (Rocha et al., 2018), as well as it was taken in cats for seven months (Zero et al., 2020). The dogs’ cadavers obtained an average score of 7.32 ± 1.63, and 75.67% of the students were in favor of using cadavers for surgical training and 80% in favor of using chemically prepared cadavers (Rocha et al., 2019).
The process of curing meat is a traditional practice in the food industry and consists of the injection of nitrite, nitrate, sodium chloride, and sugar into the meat. By a combined action, these components have a pronounced effect on the products’ characteristics and stability. Nitrite stabilizes the typical red color, improves the organoleptic characteristics, and mainly inhibits C. botulinum (Leitão, 1978).
Vacuum packaging is considered a modified atmosphere. There is a change in air pressure by reducing oxygen, thus causing a decrease in the regular respiratory activity of food and the microbial population, leading to a reduction in deterioration (Young et al., 1988). The vacuum also promotes a decline in oxidation and muscle strength values, which causes the softness of the product (Leonel, 2008).
This study aimed to determine the maximum rupture force (in Newtons - N) and the rupture elongation (in cm) of the skin in dogs’ cadavers chemically prepared with ethyl alcohol and curing salt and the evaluation of undergraduate veterinary medicine students through the practice of incision and suture of the skin.
MATERIAL AND METHODS
The dogs’ cadavers were from the Zoonosis Control Center in Ribeirão Preto, SP, in a process approved by the Municipal Legal Department (process 02.2014.000027-1). Sixteen specimens, male and female, died from causes that did not involve evident morphological alteration, such as large tumor masses or bone fractures. The animals were frozen (freezer at -18°C) after death and then transported to the Laboratory of Animal Anatomy at UNESP Jaboticabal, SP, located 50 km away.
The animals’ weight 7.87 ± 2.57, a body score between 4 and 5 on a 1 to 9 scale, considered the ideal by Laflamme (1997). They were thawed in a horizontal refrigerator at 4-6°C and trichotomized. They were then divided into two groups: group 1 for the biomechanical analysis of the skin and group 2 for the practical evaluation of cutaneous suture and incision.
In group 1, three skin fragments were collected, which became the control samples for immediate biomechanical analysis.
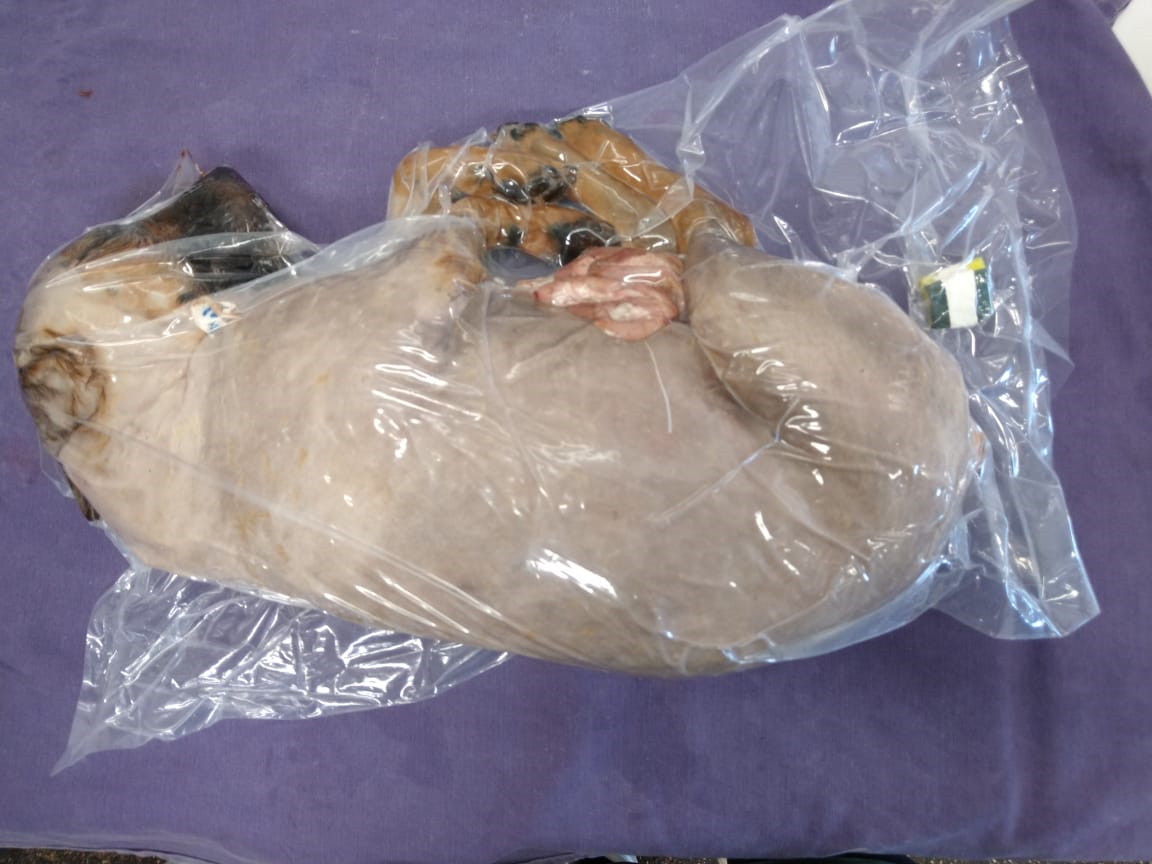
The common carotid artery was dissected for the anatomical technique, and a 40x12 cannula was inserted on it. A solution containing 200 g/L of sodium chloride, 10g/L sodium nitrite, and 10g/L of sodium nitrate (CS) was injected with a 60ml syringe (120 ml/Kg), followed by pure ethyl alcohol with 5% glycerin (EAG) (150 ml/Kg). Each corpse was placed in a plastic bag and vacuum-packed (Fig. 1) in a professional machine (Cetro® DZ Q600 DE) and kept in a horizontal refrigerator between 0 and 4°C, with a digital thermometer attached to the refrigerator’s sealing cover on the outside.
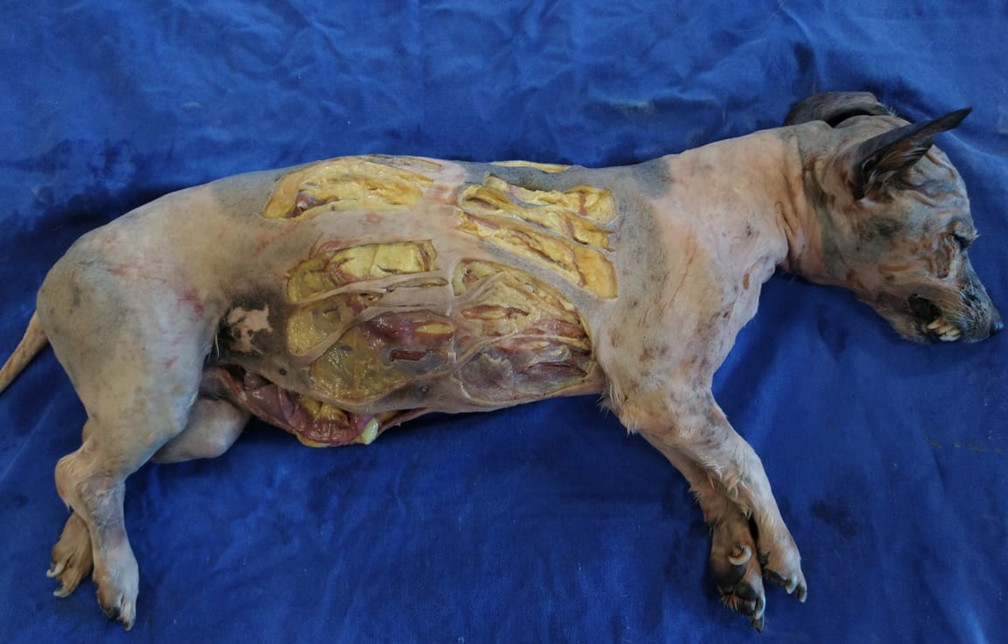
For the biomechanical analyzes of the skin, three samples were collected for seven consecutive days (breaking the vacuum packaging and re-do it again). The corpse was positioned in the right lateral decubitus and divided into four quadrants. A stainless-steel mold of 1x5 cm was placed on the side of the thorax, parallel and 5 cm from the median plane, in such a way that the first analysis (day 0) and the following analyses (days 1, 2, 3) were made on this antimere and the other four analyzes (days 4, 5, 6, 7) were made on the other antimere (Fig. 2). With a scalpel (blade number 23), the skin was excised, three sequential samples were collected in the transverse direction of the dog’s skin tension line (Fig. 2). After each analysis, the animal was placed in a plastic bag and vacuum-packed again.
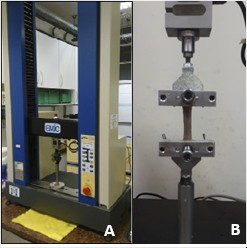
An EMIC® Universal Testing Machine - model DL-2000, present in our Department, was used to assess tissue resistance. The load cell was 500N, the load application speed was 100 mm/min, and the free space between the grips was 20 mm. The equipment belongs to the Laboratory of Surgical Anatomy of the Department of Animal Morphology and Physiology of FCAV - UNESP – Jaboticabal (Fig. 3).
For this group, statistics were performed using the software R 3.6.1 for Windows, comparing the maximum rupture force (MRF) of the skin and the rupture of elongation (RE) at all days to the control samples (D0). The MRF Cramer-Von Mises normality test and the Kruskal-Wallis test was performed. And for the RE, the Cramer-Von Mises normality test, the F test, ANOVA, and Tukey test (5%) were applied.
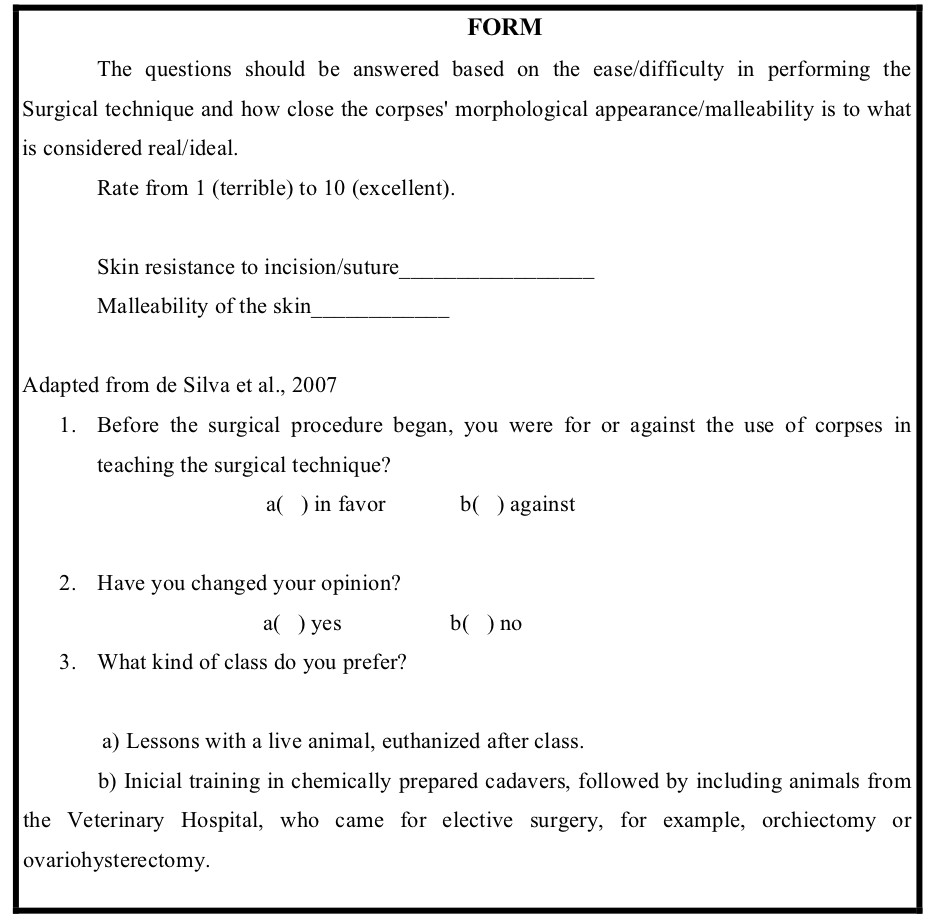
Cadavers from group 2 were chemically prepared as previously described and analyzed by 46 students from graduation from the veterinary surgical technique classes, between days 3 and 5 of conservation, which was the days with the best biomechanical results. A form was distributed to the students, which contained statements for evaluating scores related to skin malleability and incision/suture of the tissue (Fig. 4). In every class, fresh animals were taken for proper comparison with the prepared ones. Values from 1 (terrible) to 10 (excellent) were assigned to compare the animals prepared to compared to the fresh ones. The students were instructed to make an incision in the prepared animal’s skin, followed by a separate simple suture with nylon. For the statics analyses the Student’s T-test (5%) was used.
RESULTS
For group 1 the maximum rupture force (MRF) of the skin and the rupture of elongation (RE) at all days was compared to the control samples (D0), as shown in Table 1.
Table 1. Means and standard deviations of the MRF.
|
Days |
MRF |
|
D0 |
175.53±116.32 |
|
D1 |
179.90±112.18 |
|
D2 |
157.82±95.74 |
|
D3 |
141.26±92.09 |
|
D4 |
139.73±94.29 |
|
D5 |
160.78±119.41 |
|
D6 |
162.31±80.76 |
|
D7 |
152.18±114.56 |
In MRF, the Cramer-Von Mises normality test was performed (p <0.05), and P = 0.2276, so then the Kruskal-Wallis test (P = 0.8560). There was no significant difference between treatments, and data did not show normality even after undergoing BoxCox transformation.
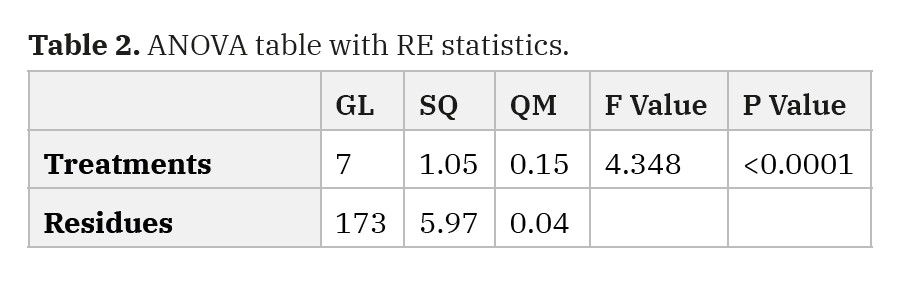
In the RE, the Cramer-Von Mises normality test (p <0.05) was applied (p=0.2145), and box cox transformation was taken. Therefore, the F test was significant (p <0.0001). Thus, the averages of treatments differ from the control, and to complement the ANOVA, the Tukey test (5%) was taken, which allowed for comparison of the means. Only days 2 and 6 were different from the control in skin biomechanics.
Table 2. ANOVA table with RE statistics.
|
GL |
SQ |
QM |
F Value |
P Value |
|
|
Treatments |
7 |
1.05 |
0.15 |
4.348 |
<0.0001 |
|
Residues |
173 |
5.97 |
0.04 |
In group 2, in which undergraduate students evaluated the animals, there was no significant difference (p=0.0517) between the scores given in malleability for fresh (9.05±1.05) or chemically prepared skin (8.45±0.83), by the 5% Student T-test. In incision, there was a significant difference (p = 0.0160) between the scores attributed in fresh (9.15±0,81) or chemically prepared skin (8.55±0.69). The same was observed in the suture, in which fresh skin obtained better evaluation by students (9.20±0,77) when compared to embalmed one (7.95±1.19), and this difference was statistically significant (p = 0.0003) by the Student´s T-test 5%.
Regarding the questions, 100% of the students favored using cadavers to perform surgical techniques. All students preferred the initial training in chemically prepared cadavers, followed by surgery classes with live animals.
Table 3. Tukey test evaluation of RE averages.
|
Days |
Mean |
|
|
D0 |
1.56±0.07 |
a |
|
D1 |
1.56±0.07 |
a |
|
D2 |
1.47±0.07 |
b |
|
D3 |
1.56±0.09 |
a |
|
D4 |
1.56±0.08 |
a |
|
D5 |
1.57±0.11 |
a |
|
D6 |
1.47±0.09 |
b |
|
D7 |
1.56±0.09 |
a |
* Means followed by different letters differ from each other (Tukey test at 5%).
DISCUSSION
The use of chemically prepared cadavers to teach surgical techniques in veterinary medicine graduation is an alternative to using live animals. Surgical training using cadavers reduces costs and increases exercise repeatability (Oliveira, 2008).
Like the ones in this research, the use of chemically prepared animals is a trend in universities worldwide and avoids the euthanasia of thousands of specimens for surgical training (Balcombe, 2000).
Surgical training on chemically prepared dogs’ cadavers proved to present excellent acceptance by undergraduate veterinary students, and even preferable to live animals as the first contact with the surgery, providing better learning (Knight, 2007), similar to the 93.29% (Silva et al., 2003) prepared with the modified Larssen solution or the 81.08% (Rocha et al.,2019) of the students that preferred to perform the first surgical training in cadavers prepared with ethyl alcohol.
All the students were satisfied with the embalmed specimens’ experience, which is consistent with another Brazilian study presenting 75.67% of approval in the use of chemically prepared cadavers for veterinary surgery practicing (Rocha et al., 2019).
The result of our research using cadavers chemically prepared with curing salts and ethyl alcohol with glycerin proved to be effective. The results were similar to those obtained in another study using the same fixative and preservative but in cat cadavers (Fração et al., 2019) and used in human cadavers for up to 12 months (Goyri-O’neill et al., 2013).
The anatomical technique used was similar to the one that used EA for fixation and 30% SCAS for preserving dogs for up to 4 months, aiming for surgical training in skin and intestines (Rocha et al., 2018), common carotid arteries (Cerqueira et al., 2017) and external jugular veins (Pelogia et al., 2018). However, the malleability and subjective evaluation of the cadavers’ color of our study was considered superior to the technique used previously according to the data in our forms.
The skin maintained its softness and color, differently from what happens when solutions with formaldehyde are used (Groscurth et al., 2001; Hayashi et al., 2016). However, there was a statistical difference compared to the fresh corpse. The malleability, incision, and suture have always been more significant than 7.95, on a scale from zero to ten (with the fresh corpse representing an almost maximum score in the evaluated criteria, considered ideal). The excellent quality of the chemically prepared dogs for surgical training is similar to the reported in soft tissues of cadavers prepared with EA for fixation, but kept in 30% SCAS tanks for up to 4 months (score 7.32 ± 1.63) (Rocha et al., 2019).
There was no statistical difference between the control and samples’ mean, except on the D2 and D6, which demonstrated that might be due to minimal collection error or grasping on the universal testing machine. Good maintenance of tissue resistance during the research and the anatomical technique maintained the skin’s biomechanical characteristics for up to 7 days, only with the cadaver vacuum-packed and refrigerated at 2-6°C. In vacuum packaging, the air is removed, inactivating aerobic bacteria, preventing product deterioration with better quality and longer shelf life (Mantilla et al., 2010). The same was reported in a study in which the EA solution combined with the 30% sodium chloride tank was used to conserve dog cadavers for up to 4 months (Rocha et al., 2018).
In a study using cats chemically prepared with the same anatomical technique used in this experiment (EAG and CS) and preserved for 90 days but with no vacuum, the MFR of the skin samples ranged from 254.19 ± 183.25N (fresh / control samples) to 234.68 ± 108.17N (Fração et al., 2019). In our study with vacuum-packed dogs, the MRF ranged from 175.53 ± 116.32 to 152.18 ± 114.56 after seven days of storage. This MRF was lower in our research because the dog’s skin is 0.5-5 mm thick, while in the cat, the thickness is 0.4-2 mm (Affolter and Moore, 1994), thus demonstrating that the cat’s skin is more elastic than the dog.
Conclusion
The anatomical technique, which uses EAG and CS, associated with vacuum packaging, has an excellent cost-benefit ratio, in addition to reduced environmental impact. Furthermore, it is an effective method for preserving the biomechanical characteristics of the skin for seven days. Also, it was observed that the technique maintains malleability, ease of incision, and suture in surgical training, which is recommended for the training and practice of veterinary surgery.
ACKNOWLEDGEMENTS
The authors wish to thank Fundação de Amparo à Pesquisa do Estado de São Paulo, FAPESP (process 2018/18567-0) and Usina São Martinho, Pradópolis, SP, Brazil.
Related articles
AFFOLTER VK, MOORE K (1994) Histologic features of normal canine and feline skin. Clin Dermatol, 12: 491-497.
BALCOMBE J (2000) The use of animals in higher education: problems, alternatives and recommendations. The Humane Society Press, Washington, DC.
CAMARGO AD, CAMARGO PC, LEAL LM, GARCIA FILHO SP, MARTINS LL, SHIMANO AC, MACHADO MRF (2014) Propriedades tensiométricas do peritônio da paca (Cuniculus paca) a fresco e conservado em glicerina 98%. Pesq Vet Bras, 34: 185-191.
CARPENTER LG, DONALD L, PIERMATTEI DL, SALMAN MD, ORTON C, NELSON AW, SMEAK DD, JENNINGS PB, TAYLOR RA (1991) A comparison of surgical training with live anesthezed dogs and cadavers. Vet Surg, 20: 373-378.
CERQUEIRA ESF, PELOGIA MES, SILVEIRA CPB, FECHIS ADS, ROCHA TASS, LAUS JL, OLIVEIRA FS (2017) Suture analysis and arterial traction test in dogs fixed on alcohol and preserved on saline solution aiming surgical practice. GARJMMS, 6: 292-295.
CORRÊA WR (2003) Isolation and identification of filamentous fungi found in anatomical pieces preserved in 10% formalin solution. Institute of Research and Development, University of the Valley of Paraíba, 59p.
FRAÇÃO VC, ZERO RC, RODRIGUES A, FERREIRA BN, FECHIS ADS, ROCHA TASS, IOZZI MT, OLIVEIRA FS (2019) Analysis of the skin of cats’ corpses chemically prepared with ethylic alcohol and curing salt aiming veterinary surgical practice - chronic effect on biomechanics and students’ evaluation. CPQ Medicine, 4: 01-08.
GOYRI-O’NEILL J, PAIS D, FREIRE FA, RIBEIRO P, O’NEILL ABA, RAMOS S, MARQUES CN (2013) Improvement of the embalming perfusion method: The innovation and the results by light and scanning electron microscopy. Acta Med Por, 26: 188-194.
GROSCURTH P, EGGLI P, KAPFHAMMER J, RAGER G J, HORNUNG P, FASEL JDH (2001) Gross Anatomy in the surgical curriculum in Switzerland: improved cadaver preservation, anatomical models, and course development. New Anat, 265: 254-256.
HAYASHI S, NAITO M, KAWATA S, QU N, HATAYAMA N, HIRAI S, ITOH M (2016) History and future of human cadaver preservation for surgical training: from formalin to saturated salt solution method. Anat Sci Int, 91: 1-7.
JANCZYK P, WEIGNER J, BECKER AL, KAESSMEYER S, PLENDL J (2011) Nitrite pickling salt as an alternative to formaldehyde for embalming in veterinary anatomy—A study based on histo- and microbiological analyses. Ann Anat, 193: 71-75.
KNIGHT A (2007) The effectiveness of humane teaching methods in veterinary education. ALTEX, 24: 91-109.
LAFLAMME D (1997) Development and validation of a body condition score system for cats: a clinical tool. Feline Practice, 25: 13-18.
LEITÃO MFF (1978) Microrganismos patogênicos na carne e derivados. ITAL, 59: 15-48.
LEONEL FR (2008) Irradiação e qualidade da carne de frango congelada e embalada a vácuo. Doctorate Thesis. Faculdade de Ciências Agrárias e Veterinárias – Unesp, Campus de Jaboticabal. Jaboticabal, SP.
MANTILLA SPS, BORGES SM, VITAL H (2010) Atmosfera modificada na conservação de alimentos. Rev Acad Ciência Agrário Ambiente, 8: 437-448.
MATHEWS KG, RILEY K, LASCELLES BDX, DERNELL WS (2010) Preparation of canine and feline cadavers for surgical laboratories. Vet Surg, 39: 224-225.
OLIVEIRA HP (2008) Situação atual do ensino da técnica cirúrgica e da clínica cirúrgica. Ciênc Vet Tróp, 11: 93-94.
PELOGIA MES, CERQUEIRA ESF, SILVEIRA CPB, ROLIM GS, FECHIS ADS, ROCHA TASS, LAUS JL, OLIVEIRA FS (2018) Suture and venous traction test analysis in dogs fixed in alcohol and preserved in saline solution. Pesq Vet Bras, 38: 1834-1837.
PAIXÃO RL (2008) Métodos substitutivos ao uso de animais vivos no ensino - Repensando o que aprendemos com os animais no ensino. Ciênc Vet Tróp, 11: 88-91.
ROCHA TASS, YANAGIHARA GR, SHIMANO AC, ROLIM GS, SANTOS CCC, FECHIS ADS, OLIVEIRA FS (2018) Biomechanical analysis of the skin and jejunum of dog cadavers subjected to a new anatomical preservation technique for surgical teaching. J Plastination, 30: 16-23.
ROCHA TASS, SANTOS CCC, IOZZI MT, DIAS RS, ZERO RC, CARDOZO MV, OLIVEIRA FS (2019) Chemically prepared dog cadavers in teaching of surgical technique - evaluation by students of a veterinary medicine course. Acta Sci Anat, 1: 136-140.
RODRIGUES H (2010) Técnicas anatômicas. GM Gráfica & Editora. Vitória-ES. 269p.
SILVA RMG, MATERA JM, RIBEIRO AACM (2003) Avaliação de ensino da técnica cirúrgica utilizando cadáveres quimicamente preservados. Revista de Educação Continuada em Medicina Veterinária e Zootecnia do CRMV-SP, 6: 1-3.
SILVA RMG, MATERA JM, RIBEIRO AACM (2004) Preservation of cadavers for surgical technique training. Vet Surg, 33: 606-608.
SILVA RMG, MATERA JM, RIBEIRO AACM (2007) New alternative methods to teach surgical techniques for veterinary medicine students despite the absence of living animals. Is that an academic paradox? Anat Hist Embryol, 36: 220-224.
YOUNG LL, REVIERE RD, COLE B (1988) Fresh meats: a place to apply modified atmospheres. Food Sci Technol, 42: 65-69.
WERDELMANN R, GERICS B (2016) Preservation of specimens for students-formaldehyde vs. salt-based fixative: 182. Anat Histol Embryol, 45: 93-94.
WHO – World Health Organization (1991) IPCS International Programme on Chemical Safety – Formaldehyde - Health and Safety Guide. n.57. Available in: <http://www.inchem.org>.
ZERO RC, SHIMANO AC, CARDOZO MV, SANTOS CCC, FECHIS ADS, ROCHA TASS, OLIVEIRA FS (2020) Cadáveres de gatos preparados químicamente para la enseñanza de técnicas quirúrgicas: análisis biomecánico de piel y yeyuno. Rev Investig Vet Perú, 31: e1617.