Diabetes mellitus is one of the main causes of death due to the complications that involve many organs such as heart, kidney, retina and others. Mesenchymal stem cells (MSCs) have been demonstrated to be effective in treatment of diabetes. This paper aims to evaluate the effect of stem cells in the treatment of diabetic nephropathy, clarifying their role in oxidative stress and inflammation. 30 adult male albino rats were equally divided into 3 groups. Control group (group I) received 1ml saline by intra-peritoneal (IP) injection. Streptozotocin-treated group (group II) received Streptozotocin (STZ) (60 mg/kg BW, I.P.) for induction of diabetes, then were sacrificed after 4 weeks. Steptozotocin + Stem cell-treated group (group III) received STZ, were left for 4 weeks, and then they were injected once intravenously with 1 million units of MSC and sacrificed after 4 weeks. Blood glucose, serum urea and creatinine were checked. The kidney sections were examined Histologically with H&E and PAS Stain and immunohistochemically for endothelial Nitric Oxide Synthase (eNOS). A morphometric study and statistical analysis were performed. DM led to increased levels of glucose, urea and creatine. Also, DM caused inflammation, degeneration and decreased eNOS immunoexpression of the kidney. The administration of MSCs improved the levels of glucose, urea and creatinine. Also, MSCs decreased the pathological changes and increased eNOS immunoexpression in the kidney. MSCs have effective therapeutic role in treating diabetic nephropathy.
Role of stem cell therapy in diabetic nephropathy in rats: biochemical, histological and immunohistochemical study
Ahmed Y. Sedeak1, Hanan D. Yassa1, Azza S. Moawad3, Abdalwakel A. Mohammed2, Gaber H. Abdelfatah1
1 Anatomy and Embryology Department, Faculty of Medicine, Beni-Suef University, Egypt
2 Anatomy and Embryology Department, Faculty of Medicine, Cairo University, Egypt
3 Histology Department, Faculty of Medicine, Beni-Suef University, Egypt
SUMMARY
Sign up or Login
INTRODUCTION
Diabetes mellitus (DM) is one of the most prevalent non-communicable metabolic diseases. Recent years have showed a sudden increase of diabetes all over the world. The International Diabetes Federation (IDF) reported that the number of diabetic populations will increase from 415 million in 2015 to 642 million by 2040 (Peng et al., 2018).
The effectiveness of MSCs was demonstrated in the treatment of diabetes and its complications, including microvascular complications such as retinopathy (Yang et al., 2010) and nephropathy (Fang et al., 2012), and macrovascular complications such as cardiomyopathy (Abdel Aziz et al., 2008).
Diabetic nephropathy is a major microvascular complication in patients with diabetes, and remains the leading cause of chronic kidney disease, accounting for approximately a half of all end-stage renal disease worldwide (Kanwar et al., 2011).
Streptozotocin (STZ) is one of antibiotics that obtained from Streptomyces achromogenes. It has been widely used for inducing experimental diabetes mellitus in a variety of animals, as it causes degeneration of pancreatic β cells causing insufficient insulin secretion (Mousa et al., 2016).
Stem cell treatment is a recent type of intervention strategy that is introduced into the damaged tissues in order to treat diseases or injuries. Many medical researchers believe that stem cell treatment has the potential to change the prospect of human diseases and alleviate suffering. The stem cells are able to proliferate and give rise to subsequent generations with variable degrees of differentiation capacities. This ability offers significant potential for the generation of tissues that can replace diseased and damaged tissues in the body (Mousa et al., 2016).
Mesenchymal stem cells (MSCs) secrete numerous factors, such as nitric oxide and prostaglandin E2, which enhance antioxidant defenses, inhibit oxidation factors and reduce necrosis of the cells (Pulavendran et al., 2010; Berardis et al., 2015). Furthermore, immune modulation of MSCs inhibits inflammatory cell proliferation, thereby exerting anti-inflammatory effects (Volarevic et al., 2014).
The goal of this study was to reveal the changes induced by diabetes on the kidney clarifying the possible therapeutic effects of MSCs through histological, immunohistochemical and biochemical studies.
MATERIALS AND METHODS
The study was carried out at the Animal House of the Faculty of Medicine, Cairo University, according to the guide lines used for the care and the use of laboratory animals (code of ethical approval is 018-61).
Experimental animals
The study was performed on 30 adult male albino rats of an average weight 150-180 g. The rats were acclimatized in the laboratory for a period of two weeks before performing the experiment. They were manipulated in cages made of metal under standard laboratory and pathogen-free environmental conditions. The rats were nourished using standard levels of the rodent water and food. These rats were equally divided into 3 groups:
Control group (group I): this group received 1ml saline by intra-peritoneal (IP) injection.
Streptozotocin-treated group (group II): this group received Streptozotocin (STZ) (60 mg/kg body weight (BW), I.P.) for induction of diabetes; then they were sacrificed after 4 weeks (Srinivasan et al., 2005).
Steptozotocin+ Stem cell-treated group (group III): this group received STZ for induction of diabetes and left for 4 weeks, then these rats were intravenously injected with 1 million units of bone marrow-derived mesenchymal stem cells (BM-derived MSCs) only for one time. After 4 weeks of being treated with stem cells, these rats were sacrificed (Ngoc et al., 2011).
Chemicals
Streptozotocin (STZ) was obtained from Sigma Company (St. Louis Mo, USA) in the form of powder solvent. Each vial of Streptozotocin powder contain 1 gram of Streptozotocin-active ingredient with the chemical name, N(Methylnitrosocarbamoyl)-α-D-glucosamine Streptozotocin.
Induction of DM: STZ (60 mg/kg BW) was dissolved in 0.1M sodium citrate buffer in the Biochemistry department, Faculty of Medicine, Cairo University. Preparation of the solution was done at pH 4.5 and then injected intravenously within 15 min. The aim of this procedure is to induce T1DM (Cesaretti et al., 2010).
Diagnosis of Diabetes: Polydipsia and Polyphagia were observed in adult rats within three days of injection of streptozotocin and this suggested DM due to destruction of β-cells of Langerhans islet cells (Bluestone et al., 2010). Diagnosis of diabetes was approved by elevation of blood glucose level (Ikebukuro et al., 2002). Rats with blood glucose levels more than 200 mg/dL were considered diabetic (Cesaretti et al., 2010).
Treatment of DM: Preparation of Labeled bone marrow-derived mesenchymal stem cells was done in the stem cell unit, Biochemistry department, Faculty of Medicine, Cairo University. These stem cells were given by single I.V. injection in a dose of 1ml of about (1×106 cells/rat) 4 weeks after confirmation of diabetes (Kajiyama et al., 2010). The treatment was allowed only to the third group suspended in 1 ml normal saline (Carr et al., 2008).
Isolation and culture of bone marrow-derived mesenchymal stem cells (BM-derived MSCs) (Jiang et al., 2010):
5-bromo-2ʹ-deoxy-uridine (BrdU) labeled MSCs were prepared at Medical Biochemistry Department, Faculty of medicine, Cairo University.
Isolated and cultivation of BM-derived MSCs were carried out for 4 weeks. Adult rats were euthanized and bilateral femora and tibias were removed under sterile conditions and placed in Dulbecco’s modified eagle medium (DMEM; Gibco/BRL). Flushing out of MSCs was performed with DMEM using a syringe fitted with a 23-guage needle. This was followed by gently pipetting bone marrow from each bone many times to separate cells.
Then the cells were washed two times using DMEM, centrifuged 2250 rpm for 15min, and cultured in DMEM supplemented with 10% fetal bovine serum (GibcoBRL), 100 U/ml penicillin G and 100mg/ml streptomycin (GibcoBRL) at 2.5x105/cm2. The cells were incubated at 37 ºC in humidified 95% air and 5% CO2.
3 days later, elimination of non-adherent cells was done. Addition of fresh complete culture medium DMEM was done and then replaced every 3 or 4 days. When the cells become 80-90% confluent over 14 days, they were harvested with 0.25% trypsin and one mmol EDTA (GibcoBRL) for 3 min at 37° C, replanted in six-well disk at 1.5x105/cm2 and again grown to near confluence. Dilution of cells was obtained by adding water 1:2 per passage to expand the culture.
Fluorescence phase-contrast microscope (Axiocam MR R3, Carl Zeiss, Germany) was used to observe the rats MSCs every 2 or 3 days.
Biochemical study
At the end of the experiment of each group, collection of blood samples were carried out using fine heparinized capillary tube. The blood samples were delivered into centrifuge tubes and the plasma was collected and used for the determination of glucose, urea and creatinine.
Anaesthesia of the rats was performed using mild ether inhalation then subsequent sacrifice of these rats was done by cervical dislocation to avoid chemical injury (Liu et al., 2013). The rats’ kidneys were excised immediately and carefully.
Histological study
The kidneys were dissected, fixed in 10% formalin overnight, processed for paraffin blocks and sectioned at 5 µm thickness. Sections of paraffin were used in:
Hematoxylin & Eosin stain (Kiernan, 2015)
PAS (Bancroft and Gamble, 2008)
Immunohistochemical study using endothelial nitric oxide synthase (eNOS) (Takahashi and Harris, 2014): Primary antibody: endothelial Nitric Oxide Synthase (eNOS) antibody, a mouse monoclonal [M221] antibody (IgG1) (Abcam Medical, Cambridge, USA, catalogue number ab76198) to eNOS and it consists of recombinant part of mouse eNOS protein which contained residues of amino acid in the C-terminal region. It is liquid and stored at -20°C in 0.05% sodium azide. Heart section was used as a standard positive control. One of the kidney sections was used as a negative control by avoiding application of the primary antibody.
Fluorescent microscopic examination
Kidney Sections of STZ+stem cell group were submitted to fluorescent microscopy examination to clarify fluorescent labeled mesenchymal stem cells (Fig. 1). It was done by detecting the Bromodeoxyuridine (BrdU) - positive cells in the sections. The sections were immunostained using mouse anti-BrdU (1:100, Neomarkers), and goat anti-mouse Ig GFITC (1:100, Kpl).
Morphometric study
Using a Leica Quin 500 (Leica Ltd, Cambridge, UK) computerized image analysis system, the morphometric studies were done for the intensity of the eNOS immunoexpression in the kidney sections.
Statistical analysis (Emsley et al, 2010):
Analysis of Statistics was done using SPSS software, version 16. All data will be expressed as mean ± SD. One way analysis of variance (ANOVA) test will be used for comparison between rat groups. The results of Statistics were considered significant when the p-values were < 0.05.
RESULTS
No deaths were detected in rats.
Biochemical results
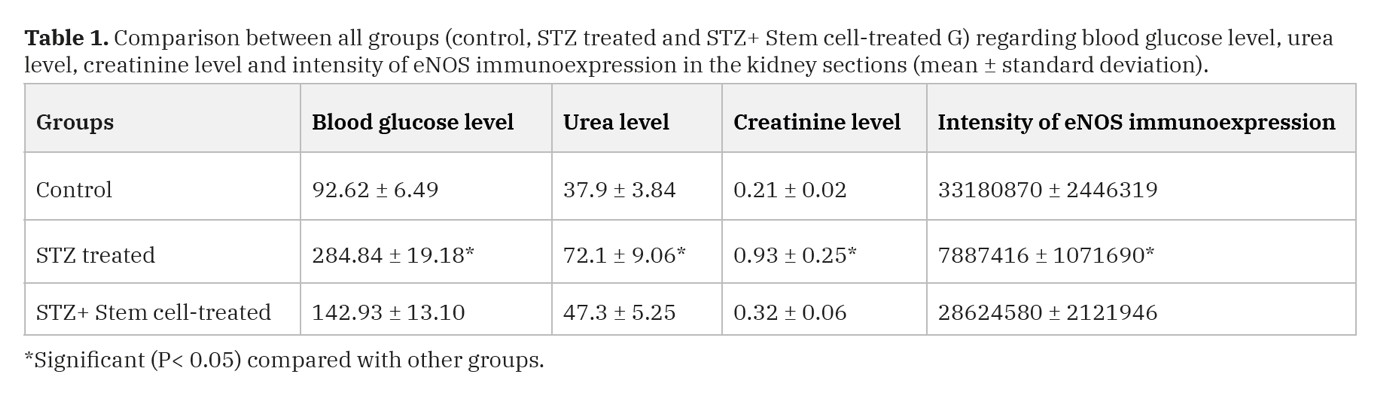
The mean values of glucose, urea and creatinine levels of STZ group were significantly increased as compared with the control group (P-value < 0.05) (Table 1).
The mean value of glucose, urea and creatinine levels of STZ+ Stem cell-treated group were significantly decreased as compared with the STZ treated group (P-value < 0.05) (Table 1).
Histological results
H and E stain results (Figs. 5, 6)
In the control group, H&E-stained sections of the kidneys revealed normal renal architecture in the form of Malpighian corpuscles formed of a glomerulus surrounded by Bowmanיs capsule, proximal and distal convoluted tubules (Fig. 2).
In the STZ-treated group, renal sections showed hypertrophic glomeruli and obliteration of the capsular spaces (Fig. 3). Renal sections also revealed cytoplasmic vacuolization of the tubular cells (Figs. 3, 4). Multiple tubules with intraluminal acidophilic masses and congested blood vessels were demonstrated (Fig. 4). There was massive mononuclear cellular infiltration (Fig. 3).
The kidneys of STZ+ Stem cell-treated group revealed normal Malpighian corpuscles formed of a glomerulus surrounded by Bowman’s capsule corpuscle and normal tubules whereas some tubules were dilated (Fig. 5).
PAS stain results (Fig. 6)
Using PAS stain, the control kidneys displayed the parietal layer of the Bowman’s capsule, the basement membrane of renal tubule and the brush border of the proximal convoluted tubule. However, in STZ-treated group, there was thickening of parietal layer of the Bowman’s capsule, as well as basement membrane of the renal tubules. Partial loss of brush border of some proximal convoluted tubules was detected. While, in STZ+ Stem cell-treated group, there was thin parietal layer of the Bowman’s capsule and basement membrane of renal tubule. Brush border of proximal convoluted tubules could be seen mostly intact.
Immunohistochemical results (Fig. 7)
Immunohistochemical staining of the kidneys for (eNOS) demonstrated positive eNOS immunoexpression in the glomerular vascular endothelial cells. However, in STZ-treated group, immunohistochemical staining of the kidneys for (eNOS) demonstrated less intensity of the eNOS immunoexpression in the glomerular vascular endothelium in comparison with the control group. While the STZ+ Stem cell-treated group showed increased intensity of the eNOS immunoexpression.
Morphometric results
The intensity of eNOS immunoexpression in STZ group was significantly decreased as compared with the control group (P-value < 0.05) (Table 1).
The intensity of eNOS immunoexpression in STZ+stem cell group showed a significant increase as compared with the STZ treated group (P-value < 0.05) (Table 1).
DISCUSSION
In the present work, the diabetic rats developed diabetic nephropathy detected biochemically and histologically. Regarding the biochemical results of the kidney, renal dysfunction is detected by elevated serum urea and creatinine. Similar to the current work, Abdel Aziz et al., (2014) reported that one of the most sensitive and dramatic indicators of kidney injury is the increase of creatinine and urea level in the circulation following STZ administration.
The results of the current study are in accordance with Kaur et al. (2015), who reported that the single dose administration of STZ in rats has been shown to produce diabetic nephropathy (DN) after 6 weeks, and stated that the serum creatinine levels were noted to be increased markedly in STZ treated rats on day 42nd as compared to the control group. Also, Lu et al. (2007) and Shaker et al. (2015) reported that urea and creatinine levels were increased in DN rat model induced by streptozotocin.
Multiple mechanisms contribute to the development and outcomes of diabetic nephropathy such as an interaction between hyperglycemia-induced metabolic and hemodynamic changes and genetic predisposition, which sets the stage for kidney injury (Li et al., 2018).
Another theory was detected by Mousa et al. (2016) who postulated that the elevation in serum urea and creatinine of STZ diabetic animals could be attributed to the functional and/or morphological changes in the kidneys.
However, Sriram and Subramanian (2011) showed that the increased concentrations of urea and creatinine in blood was due to diabetic oxidative stress and impaired balance of nitrogen coupled with lowered protein synthesis.
The current work detected that administration of BM-MSCs in diabetic rats showed a significant improvement of kidney functions (urea and creatinine) as compared with diabetic group. This was agreed with Mousa et al. (2016) and Humphreys and Bonventre (2008), who detected an improvement of kidney functions after administration of stem cells, and this may be due to homing of MSCs to injured tissue causing its regeneration by its direct differentiation ability or by the paracrine factors released by MSCs. In agreement of the current study, the results of Semedo et al. (2009b) showed a significant improvement in kidney function in the diabetic group treated intravenously with a single dose of one million of MSCs per rat compared to DN group.
Abdel Aziz et al. (2014) reported that the administration of MSCs led to the amelioration of some functional parameters such as serum creatinine and urea levels. They referred this improvement in kidney function in MSCs-treated group to their paracrine action via different growth factors such as VEGF, TGF-β and TNF-α and antiapoptotic effects via Bax and Bcl2 genes.
Regarding the histological results, STZ injection caused structural alterations in the renal glomeruli which appeared hypertrophic with narrowed or obliterated Bowman`s space. These findings were similar to that of Shaker et al. (2015) and Zhou et al. (2009) who reported that kidney samples of diabetic nephropathy group showed exaggerated mesangial proliferation and thickened basement membrane.
Additionally, Patel et al. (2009) found that DM led to an elevation in oxidative damage inducing considerable injury in the glomeruli, disorders in matrix protein synthesis and increase in transforming growth factors-beta (TGF-β). However, Takahashi and Harris (2014) found that the diabetic nephropathic changes in the form of glomerular hypertrophy, mesangial expansion and nodular glomerulosclerosis were due to not only oxidative stress but also due to insulin resistance, hyperinsulinemia and hyperlipidemia.
In the present work, diabetic nephropathy was characterized by degenerated renal tubular cells with intraluminal acidophilic hyaline material that considered as hyaline casts. Moreover, there was a significant interstitial mononuclear cellular infiltration. This is in agreement with results reported by (Mundt and Shanahan, 2011).
These findings were analogous to the findings of Li et al. (2018), who indicated that renal morphologic abnormalities in DN rats including tubular degeneration, dilatation and protein cylinders at 8 weeks after STZ injection, confirming diabetic renal injury.
In the current study, light microscopic examination of the renal sections revealed the regaining of the normal appearance of most of the renal tissue including glomeruli, proximal and distal convoluted tubules in mesenchymal stem cell treated group after STZ administration. Comparable findings were observed by Asanuma et al. (2011) and Nagaishi et al. (2016) who revealed the therapeutic use of stem cells in diabetic nephropathy.
Many postulations were suggested to explain the mechanism of stem cells therapy in diabetic nephropathy. MSCs protects against diabetic nephropathy by restoring the biochemical alterations as well as inhibition of oxidative stress and pro-inflammatory gene expression levels suggesting a potential clinical use of MSCs to prevent the onset and progression of diabetic nephropathy according to Fang et al. (2012).
Another explanation is that the use of MSCs in type 1 diabetes (T1D) was based upon their immunoregulatory properties, which may help to rescue peripheral tolerance toward pancreatic β cells by reshaping the immune response and blocking their damage by autoreactive T cells (Fiorina et al., 2011). A study made by Lee et al. (2006) mentioned that in diabetic mice, MSCs homed and promoted repair of pancreatic islets and renal glomeruli. The results raised the possibility that MSCs may be useful in enhancing insulin secretion and perhaps improving the renal lesions that develop in patients with diabetes mellitus.
Antioxidant effect of MSCs was suggested to be mediated through modulation of pathways associated with the activation of antioxidant pathways such as superoxide dismutase and glutathione peroxidase (Lanza et al., 2009). Transplantation of MSCs decreases inflammatory response of kidney in diabetic nephropathy. This leads to modulation of the inflammation via increasing IL10 cytokine and delays the progression of diabetic nephropathy. This beneficial effect is thought to be due to the anti-inflammatory factors such as IL10 produced by MSCs that are secreted in a paracrine fashion (Semedo et al., 2009b).
In the present work, PAS-stained kidney sections revealed marked thickening of parietal layer of Bowman`s capsule. The basement membranes of proximal and distal convoluted tubules were also thickened with loss of apical brush border of some proximal convoluted tubules. These results were in compliance with the finding of many researchers such as Wang et al. (2013), Nagaishi et al. (2016) and Li et al. (2018).
In STZ+ Stem cell-treated group, there were regaining the thin parietal layer of Bowman`s capsule, thin basement membrane of renal tubule and intact brush border of proximal convoluted tubules.
In the current work, the eNOS immunoexpression is detected in the glomerular endothelial cells of the kidneys of the control group. The glomerular endothelium of diabetic kidneys showed a significant decrease in the intensity of the eNOS immunoexpression compared to the endothelium of control kidneys. Also, there was a significant increase in the intensity of the eNOS immunoexpression in the glomerular endothelial cells of the kidneys in the STZ +stem cell treated group compared to the diabetic group. These findings were also detected by Aktug et al. (2012), who found that eNOS expression in the glomerular endothelial cells and the visceral and parietal layers of the capsule were stronger in the controls when compared to the diabetic group. According to their results, hyperglycemia inhibits eNOS leading to reduced nitric oxide production in endothelial cells. Inhibition of eNOS was correlated with glomerular cell loss due to apoptosis.
Animal models have explored the mechanisms by which the eNOS deficiency causes advanced DN and provided many new insights into the pathogenesis of DN (Takahashi and Harris, 2014). Hyperglycemia down regulates the expression and activity of eNOS and decreases the bioavailability of NO, which aggravates diabetic nephropathy according to (Casey et al., 2004).
Oxidative stress appears to be the most important pathogenic factor in underlying diabetic complications (Dave and Kalia, 2007). Higher levels of reactive oxygen species can induce the production of inflammatory cytokines in the kidney which enhances DN progression. One of the sources of inflammatory cytokines is bone marrow-derived cells that excessively infiltrate the kidneys fusing with the tubular epithelial cells causing parenchymal cells to produce cytotoxic tumor necrosis factor alpha (TNF-α) and caspase-3 which leads to degeneration and apoptosis in STZ-induced diabetes (Nagaishi et al., 2016).
Conclusion
MSCs transplantation can improve the levels of blood glucose and kidney functions (urea and creatinine) in STZ-induced diabetic rats. Also stem cells ameliorate the pathological changes of the kidneys in diabetic rats. Thus, stem cells provided a new line of treatment strategies in diabetes and diabetic nephropathy.
Related articles
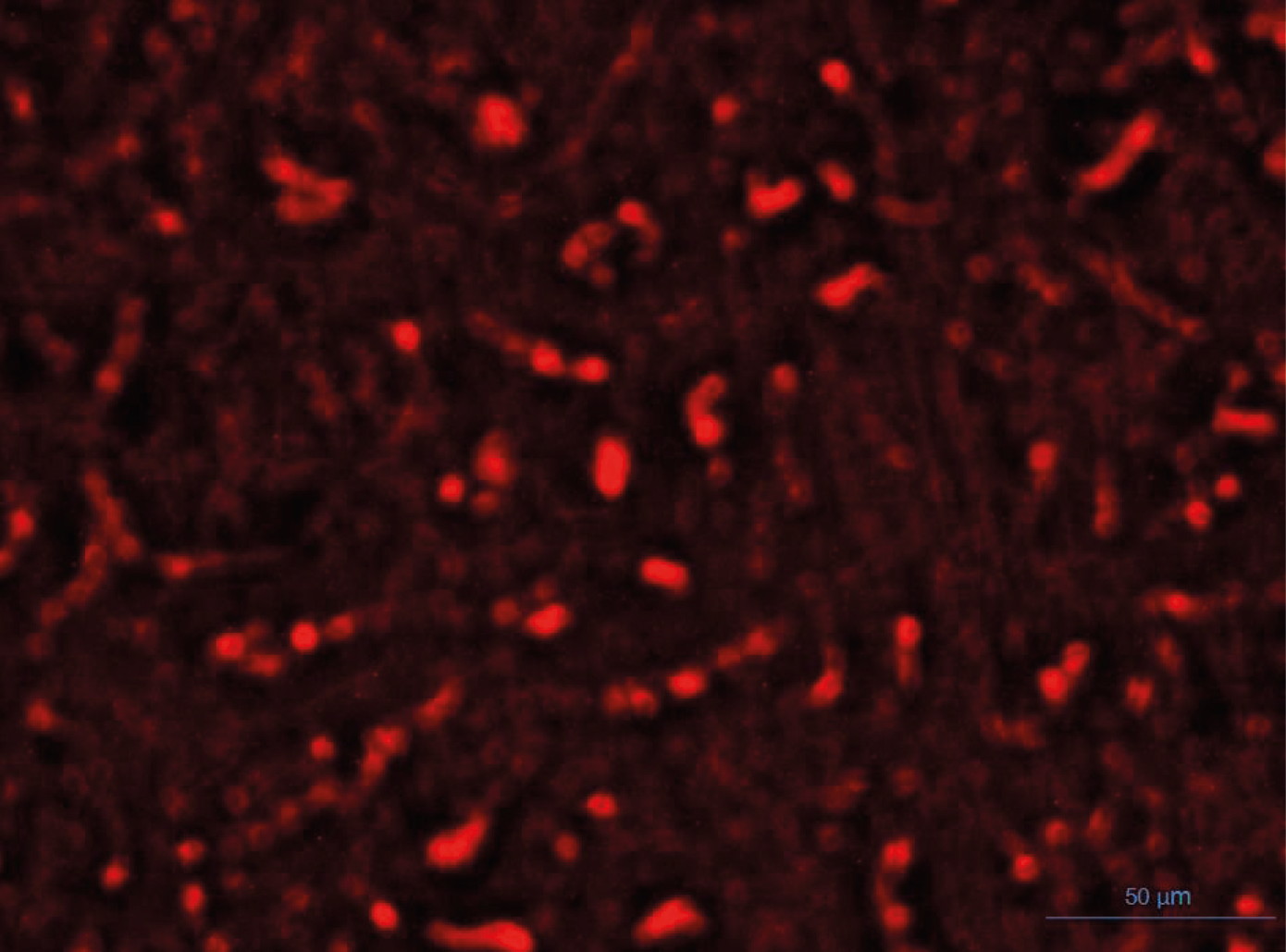 Fig. 1.- Fluorescent microscopic kidney section of the STZ+MSCs group shows fluorescent illumination of fluorescent-labeled mesenchymal stem cells. Scale bar = 50 µm.
Fig. 1.- Fluorescent microscopic kidney section of the STZ+MSCs group shows fluorescent illumination of fluorescent-labeled mesenchymal stem cells. Scale bar = 50 µm.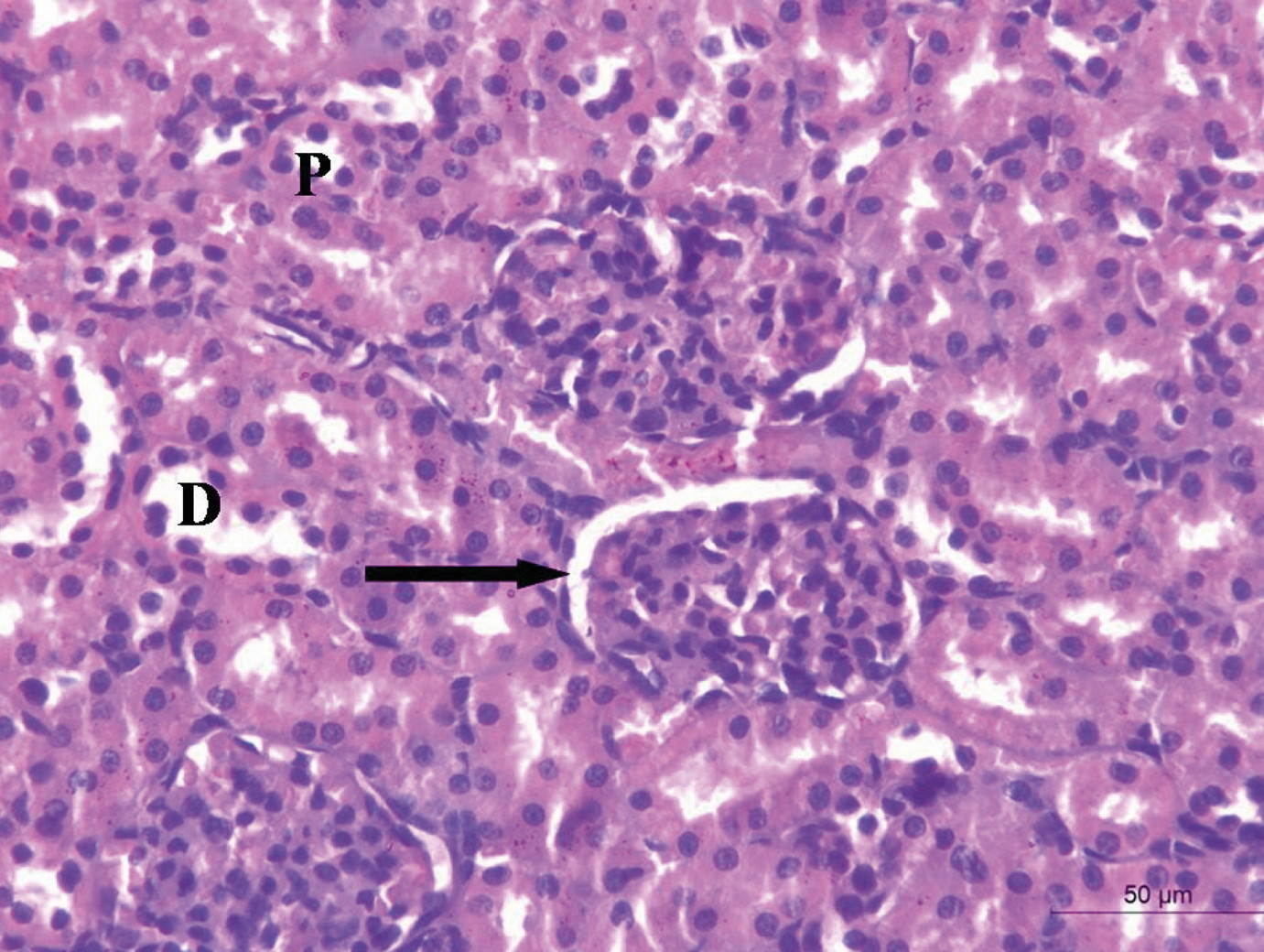 Fig. 2.- A renal cortical section of the control group shows normal Malpighian renal corpuscle formed of a glomerulus surrounded by Bowmanיs capsule (arrow), proximal convoluted tubule (P) and distal convoluted tubule (D). Scale bar = 50 µm.
Fig. 2.- A renal cortical section of the control group shows normal Malpighian renal corpuscle formed of a glomerulus surrounded by Bowmanיs capsule (arrow), proximal convoluted tubule (P) and distal convoluted tubule (D). Scale bar = 50 µm.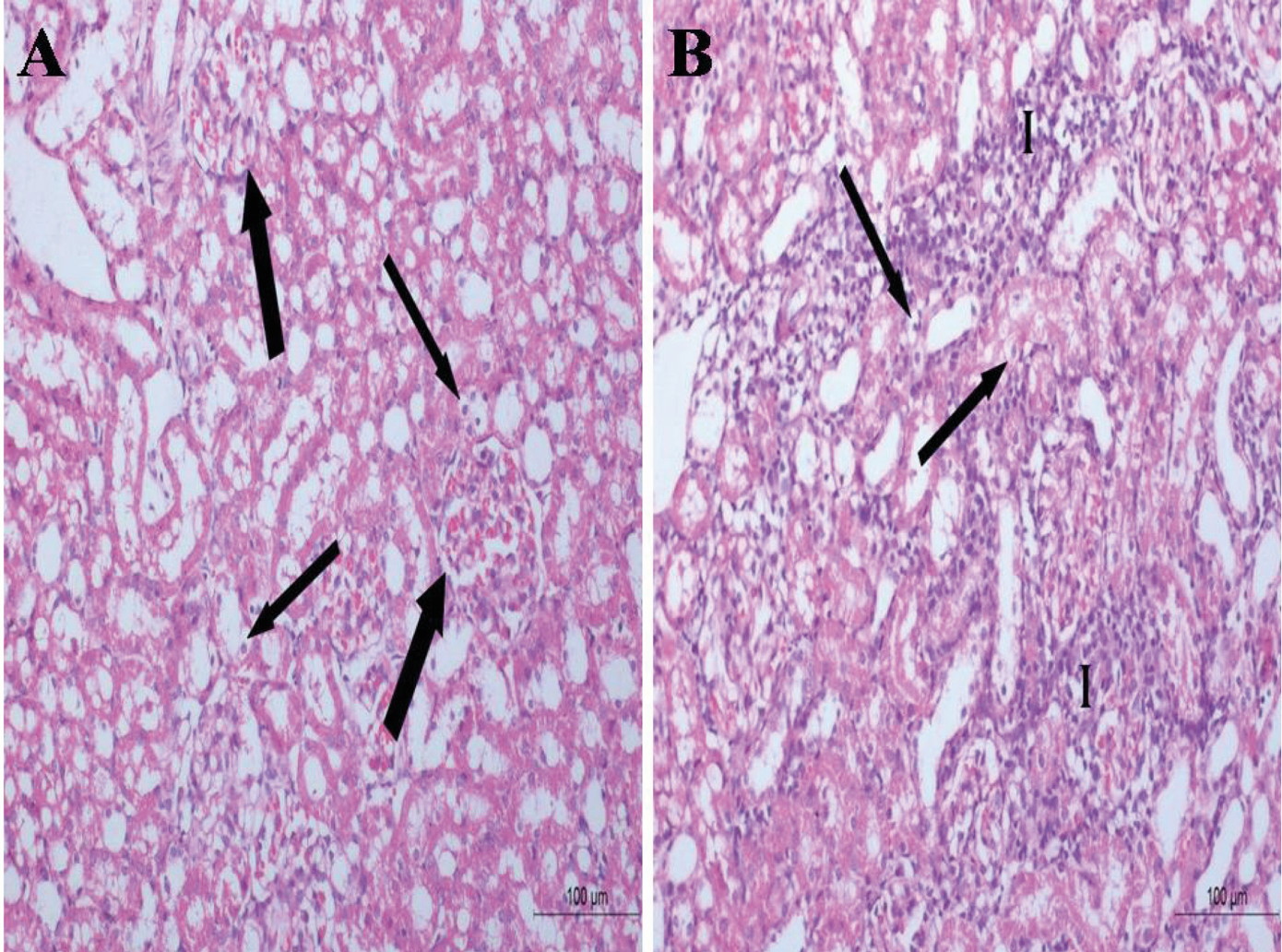 Fig. 3.- Renal cortical sections of the STZ treated group. (A) hypertrophic glomeruli and obliteration of the capsular spaces (thick arrow) and cytoplasmic vacuolation of the renal tubules (thin arrows). (B) massive mononuclear cellular infiltration (I) and cytoplasmic vacuolizations of the tubular cells (arrows). Scale bars = 100 µm.
Fig. 3.- Renal cortical sections of the STZ treated group. (A) hypertrophic glomeruli and obliteration of the capsular spaces (thick arrow) and cytoplasmic vacuolation of the renal tubules (thin arrows). (B) massive mononuclear cellular infiltration (I) and cytoplasmic vacuolizations of the tubular cells (arrows). Scale bars = 100 µm.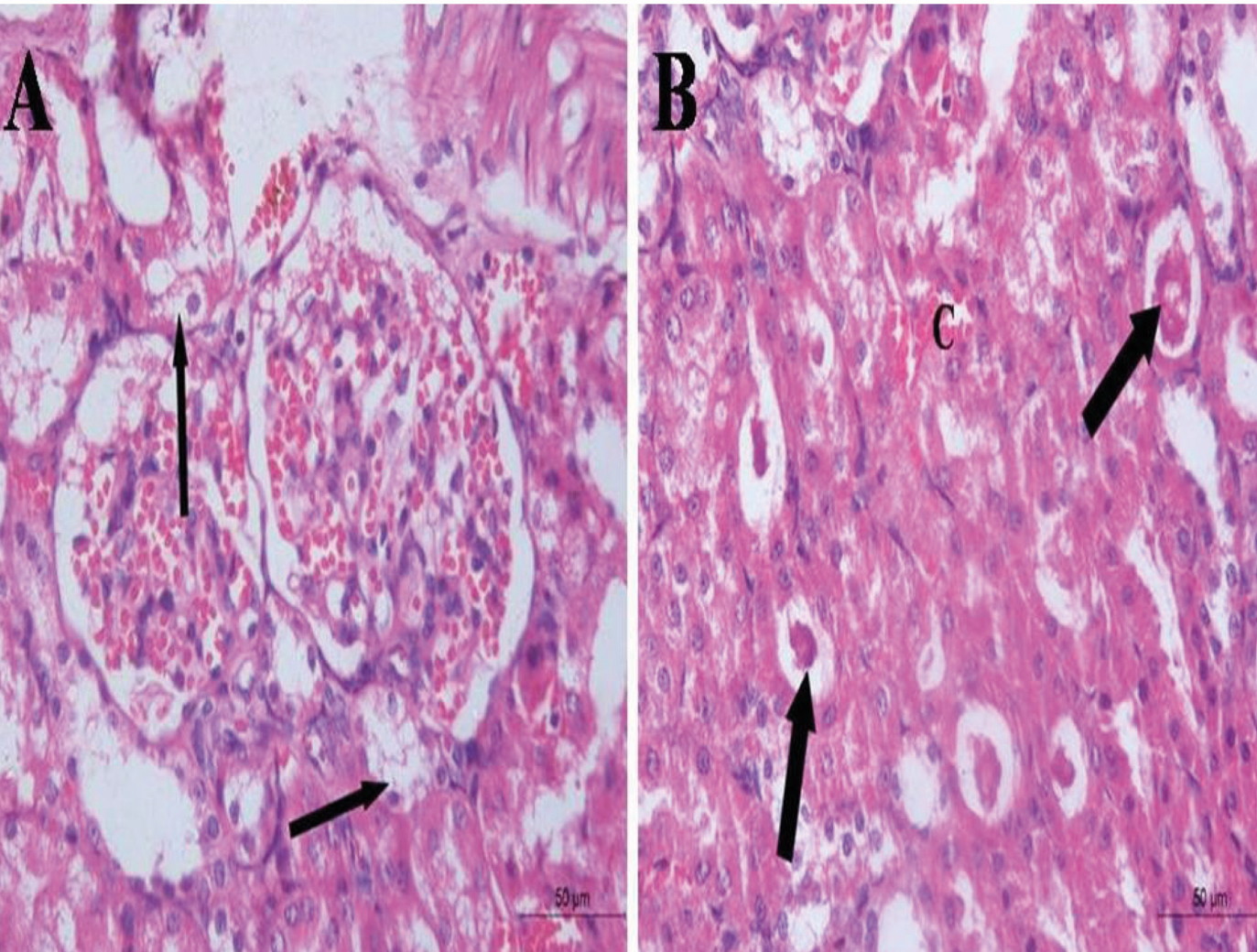 Fig. 4.- Renal cortical section of the STZ treated group. (A) cytoplasmic vacuolization of the tubular cells (arrows). (B) multiple tubules with intraluminal acidophilic masses (thick arrows) and congested blood vessels (C). Scale bars = 50 µm.
Fig. 4.- Renal cortical section of the STZ treated group. (A) cytoplasmic vacuolization of the tubular cells (arrows). (B) multiple tubules with intraluminal acidophilic masses (thick arrows) and congested blood vessels (C). Scale bars = 50 µm. Fig. 5.- Renal cortical section of the STZ+ stem cell treated group shows apparently normal Malpighian corpuscle (thick arrow) and tubules (thin arrows) and some dilated tubules (arrow heads). Scale bar = 50 µm.
Fig. 5.- Renal cortical section of the STZ+ stem cell treated group shows apparently normal Malpighian corpuscle (thick arrow) and tubules (thin arrows) and some dilated tubules (arrow heads). Scale bar = 50 µm.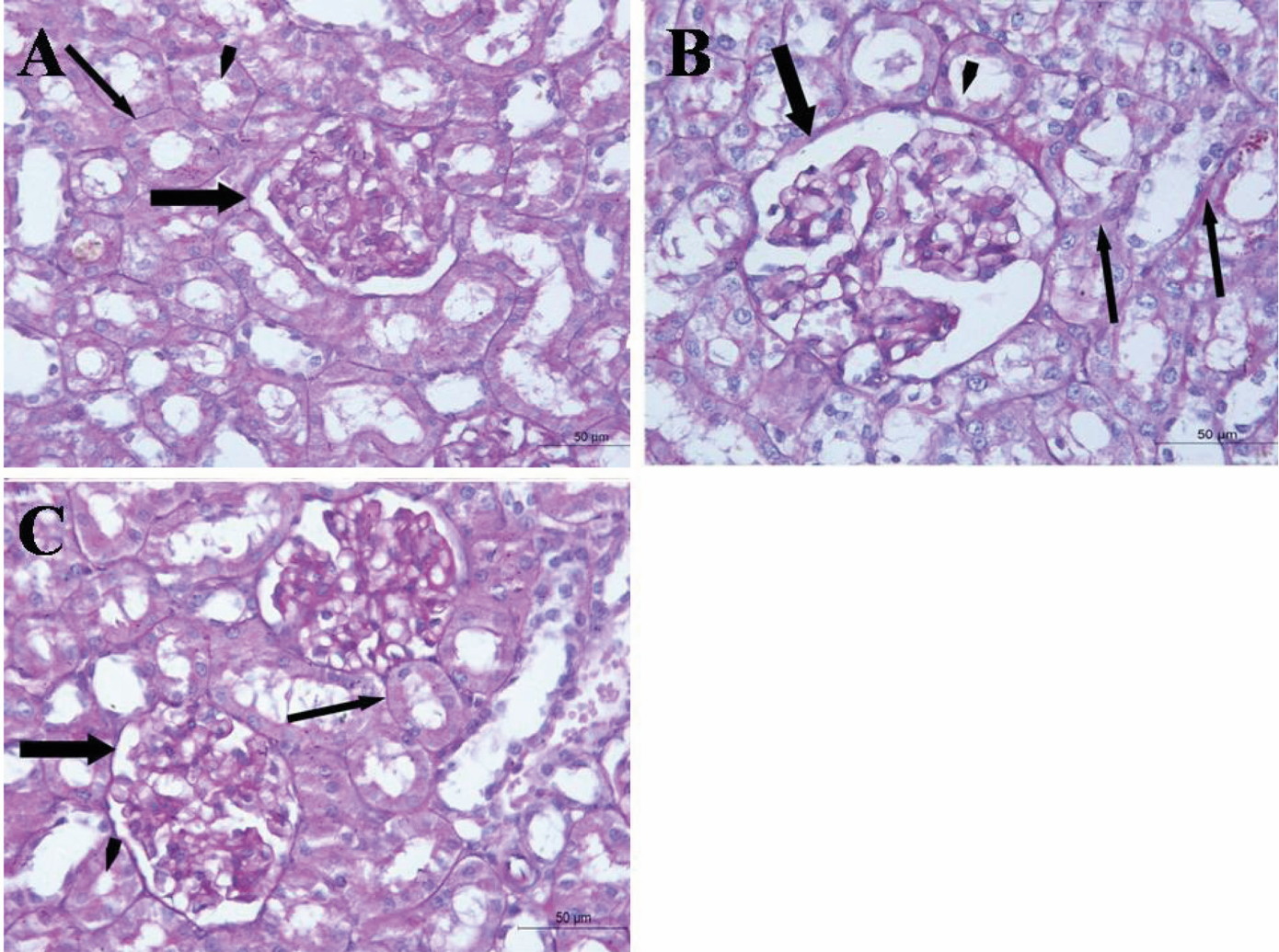 Fig. 6.- PAS-stained renal cortical sections of control (A), STZ treated (B) and STZ+ stem cell treated (C) groups. (B) shows in comparison with (A) thickening of parietal layer of Bowman’s capsule (thick arrow) as well as basement membrane of renal tubules (thin arrows). Partial loss of brush border of some proximal convoluted tubules could be seen (arrowhead). (C) shows thin parietal layer of Bowman`s capsule and basement membrane of renal tubule. Brush border of proximal convoluted tubules could be detected mostly intact. Scale bars = 50 µm.
Fig. 6.- PAS-stained renal cortical sections of control (A), STZ treated (B) and STZ+ stem cell treated (C) groups. (B) shows in comparison with (A) thickening of parietal layer of Bowman’s capsule (thick arrow) as well as basement membrane of renal tubules (thin arrows). Partial loss of brush border of some proximal convoluted tubules could be seen (arrowhead). (C) shows thin parietal layer of Bowman`s capsule and basement membrane of renal tubule. Brush border of proximal convoluted tubules could be detected mostly intact. Scale bars = 50 µm.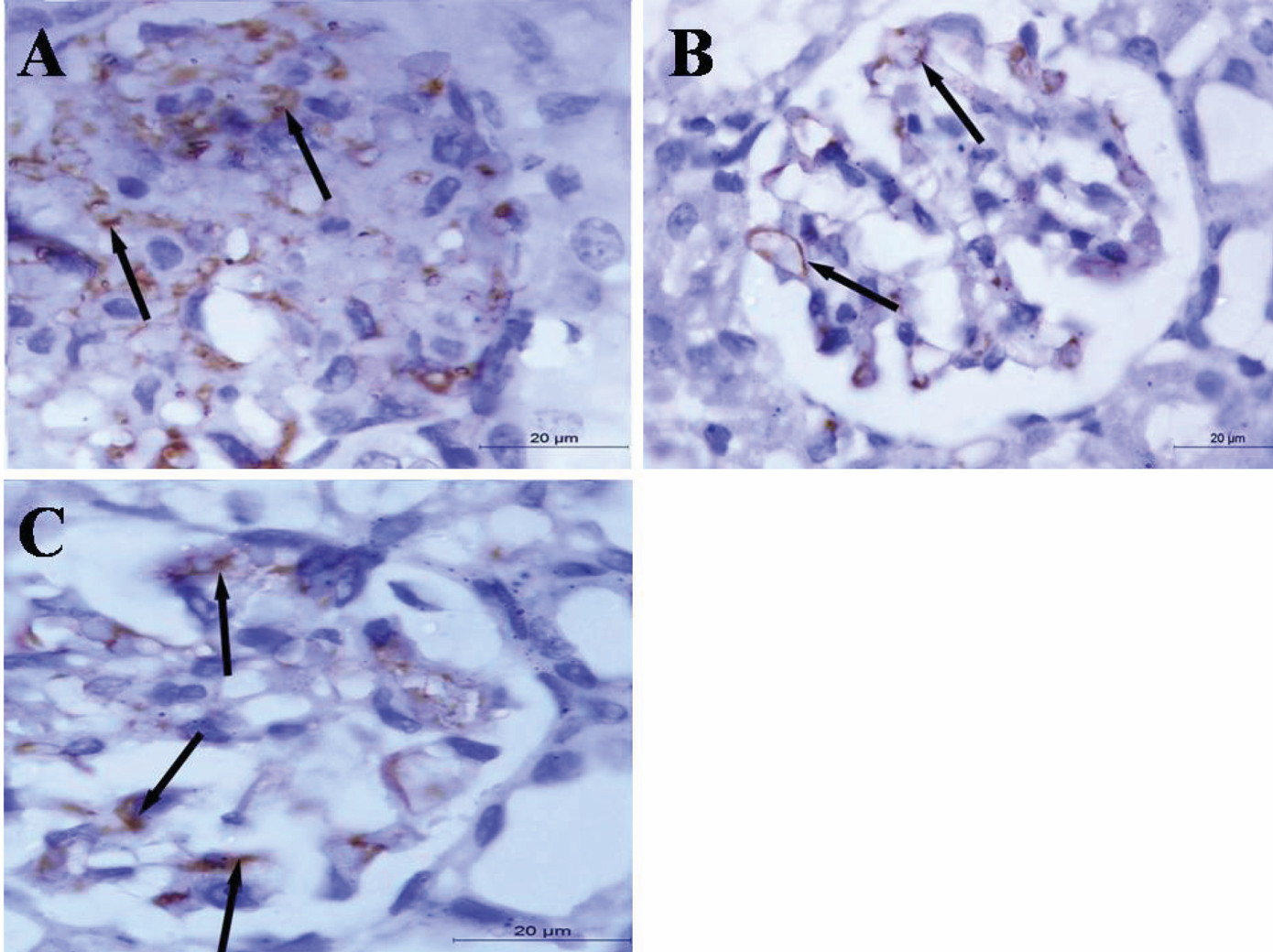 Fig. 7.- eNOS immunostained renal sections of control (A), STZ treated (B) and STZ+ stem cell treated (C). (A) shows brown coloration (positive eNOS immunoexpression) in the glomerular vascular endothelium (arrows). (B) shows decreased intensity of the eNOS immunoexpression while (C) shows an increase of the intensity. Scale bars = 20 µm.
Fig. 7.- eNOS immunostained renal sections of control (A), STZ treated (B) and STZ+ stem cell treated (C). (A) shows brown coloration (positive eNOS immunoexpression) in the glomerular vascular endothelium (arrows). (B) shows decreased intensity of the eNOS immunoexpression while (C) shows an increase of the intensity. Scale bars = 20 µm.ABDEL AZIZ MT, EL-ASMAR MF, HAIDARA M, ATTA HM, ROSHDY NK, RASHED LA, SABRY D, YOUSSEF MA, ABDEL AZIZ AT, MOUSTAFA M (2008) Effect of bone marrow-derived mesenchymal stem cells on cardiovascular complications in diabetic rats. Med Sci Monit, 14(11): BR 249-255.
ABDEL AZIZ MT, WASSEF MA, AHMED HH, RASHED L, MAHFOUZ S, ALY MI, HUSSEIN RE, ABDEL AZIZ M (2014) The role of bone marrow derived-mesenchymal stem cells in attenuation of kidney functions in rats with diabetic nephropathy. Diabetology Metab Synd, 6: 34-44.
AKTUG H, CETINTAS VB, KOSOVA B, OLTULU F, DEMIRAY SB, CAVUSOGLU T, AKARCA SO, YAVASOGLU A (2012) Dysregulation of NOS activity and Bcl-2&caspase-3 gene expression in renal tissue of STZ-induced diabetic rats. Turk J Med Sci, 42(5): 830-838.
ASANUMA H, VANDERBRINK BA, CAMPBELL MT, HILE KL, ZHANG H, MELDRUM DR, MELDRUM KK (2011) Arterially delivered mesenchymal stem cells prevent obstruction-induced renal fibrosis. J Surg Res, 168: e51-e59.
BANCROFTJ, GAMBLE M (2008) Theory and Practice of Histological Techniques. 5th ed. Churchill-Livingstone, London, Edinburgh, New York, Philadelphia, St Louis, Sydney and Toronto, pp 126-127, 150, 171, 601-612.
BERARDIS S, SATTWIKA D, SOKAL E (2015) Use of mesenchymal stem cells to treat liver fibrosis. World J Gastroenterol, 3: 742-758.
BLUESTONE JA, HEROLD K, EISENBARTH G (2010) Genetics, pathogenesis and clinical interventions in type 1 diabetes. Nature, 464: 1293-1300.
CARR CA, STUCKEY DJ, TATTON L (2008) Bone marrow-derived stromal cells home to and remain in the infarcted rat heart but fail to improve function: an in vivo cine-MRI study. Am J Physiol Heart Circ Physiol, 295(2): H533.
CASEY GR, JOYCE M, NAGLE RG, CHEN G, BOUCHIER-HAYES D (2004) Pravastatin modulates early diabetic nephropathy in an experimental model of diabetic renal disease. J Surg Res, 123: 176-181.
CESARETTI ML, GINOZA M, RIBEIRO AB, KOHLMANN OJ (2010) Systemic hemodynamic and left ventricular function of diabetic-induced hypertensive rats. Arq Bras Endocrinol Metabol, 54(9): 842-851.
DAVE GS, KALIA K (2007) Hyperglycemia induced oxidative stress in type-1 and type-2 diabetic patients with and without nephropathy. Cell Mol Biol, 53(5): 68-78.
EMSLEY R, DUNN G, WHITE IR (2010) Mediation and moderation of treatment effects in randomized controlled trails of complex interventions. Stat Methods Med Res, 19: 237-270.
FANG Y, TIAN X, BAI SH, JUN FAN J, HOU W, TONG H, LI D (2012) Autologous transplantation of adipose-derived mesenchymal stem cells ameliorates streptozotocin-induced diabetic nephropathy in rats by inhibiting oxidative stress, pro-inflammatory cytokines and the p38 MAPK signaling pathway. Int J Mol Med, 30: 85-92.
FIORINA P, VOLTARELLI J, ZAVAZAVA N (2011) Immunological applications of stem cells in type 1 diabetes. Endocr Rev, 32(6): 725-754.
HUMPHREYS BD, BONVENTRE JV (2008) Mesenchymal stem cells in acute kidney injury. Annu Rev Med, 59: 311-325.
IKEBUKURO K, ADACHI Y, YAMADA Y, FUJIMOTO S, SEINO Y, OYAIZU H (2002) Treatment of streptozotocin-induced diabetes mellitus by transplantation of islet cells plus bone marrow cells via portal vein in rats. Transplantation, 73(4): 512-518.
JIANG TS, CAI L, JI WY, HUI YN, WANG YS, HU D, ZHU J (2010) Reconstruction of the corneal epithelium with induced marrow mesenchymal stem cells in rats. Mol Vis, 16: 1304-1316.
KAJIYAMA H, HAMAZAKI TS, TOKUHARA M, MASUI SH, OKABAYASHI K, OHNUMA K, YABE SH, YASUDA K, ISHIURA SH, OKOCHI H, ASASHIMA M (2010) Pdx1-transfected adipose tissue-derived stem cells differentiate into insulin-producing cells in vivo and reduce hyperglycemia in diabetic mice. Int J Dev Biol, 54: 699-705.
KANWAR YS, SUN L, XIE P, LIU FY, CHEN S (2011) A glimpse of various pathogenetic mechanisms of diabetic nephropathy. Annu Rev Pathol, 6: 395-423.
KAUR M, SACHDEVA S, BEDI O, KAUR T, KUMAR P (2015) Combined effect of hydrogen sulphide donor and losartan in experimental diabetic nephropathy in rats. J Diabetes Metab Dis, 14: 63.
KIERNAN JA (2015) Staining with dyes in one or two colors. In: Histological and Histochemical Methods: Theory and Practice, 5th ed. Scion Publishing Ltd., Vantage Business Park, Bloxham Road, Banbury, UK, pp 137-169.
LANZA C, MORANDO S, VOCI A, CANESI L, PRINCIPATO MC, SERPERO LD, MANCARDI G, UCCELLI A, VERGANI L (2009): Neuroprotective mesenchymal stem cells are endowed with a potent antioxidant effect in vivo. J Neurochem, 110: 1674-1684.
LEE RH, SEO MJ, REGER RL, SPEES JL, PULIN AA, OLSON SD, PROCKOP DJ (2006) Multipotent stromal cells from human marrow home to and promote repair of pancreatic islets and renal glomeruli in diabetic NOD/SCID mice. Proc Natl Acad Sci USA, 103: 17438-17443.
LIU S, ZHAO C, YANG C, LI X, HUANG H, LIU N, LI S, WANG X, LIU J (2013) Gambogic acid suppresses pressure overload cardiac hypertrophy in rats. Am J Cardiovasc Dis, 3(4): 227-238.
LI Y, LIU J, LIAO G, ZHANG J, CHEN Y, LI L, LIU F, CHEN B, GUO G, WANG C, YANG L, CHENG J, LU Y (2018) Early intervention with mesenchymal stem cells prevents nephropathy in diabetic rats by ameliorating the inflammatory microenvironment. Int J Mol Med, 41: 2629-2639.
LU Q, YIN XX, WANG JY, GAO YY, PAN YM (2007) Effects of Ginkgo biloba on prevention of development of experimental diabetic nephropathy in rats. Acta Pharmacol Sin, 28: 818-828.
MOUSA F, ABDEL AZIZ KK, ABDEL GAWAD H, MAHMOUD SS, ELGAMEL MS (2016) Bone marrow-derived mesenchymal stem cells infusion ameliorates hyperglycemia, dyslipidemia, liver and kidney functions in diabetic rats. Int J Sci Res (IJSR), 5(2): 1624-1631.
MUNDT L, SHANAHAN K (2011) Graff’s Textbook of Routine of Urin analysis and Body Fluids, 2nd edition, Lippincott Williams & Wilkins, Philadelphia.
NAGAISHI K, MIZUE Y, CHIKENJI T, OTANI M, NAKANO M, KONARI N, MINEKO FM (2016) Mesenchymal stem cell therapy ameliorates diabetic nephropathy via the paracrine effect of renal trophic factors including exosomes. Sci Rep, 6: 34842.
NGOC K, VAN PHUC P, NHUNG T, THUY D, NGUYET M (2011) Improving the efficacy of type 1 diabetes therapy by transplantation of immunoisolated insulin-producing cells. Human Cell, 24: 86-95.
PATEL SS, SHAH RS, GOYAL RK (2009) Antihyperglycemic, antihyperlipidemic and antioxidant effect of Dihar, a polyherbal an ayurvedic formulation in streptozotocin induced diabetic rat. Int J Exp Biol, 471: 564-570.
PENG BY, DUBEY NK, MISHRA VK, TSAI FC, DUBEY R, DENG WP, WEI HJ (2018) Addressing stem cell therapeutic approaches in pathobiology of diabetes and its complications. J Diabetes Res, 2018: 7806435.
PULAVENDRAN S, VIGNESH J, ROSE C (2010) Differential anti-inflammatory and anti-fibrotic activity of transplanted mesenchymal vs. hematopoietic stem cells in carbon tetrachloride induced liver injury in mice. Int Immunopharmacol, 4: 513-519.
SEMEDO P, PALASIO CG, OLIVEIRA CD, FEITOZA CQ, GONCALVES GM, CENEDEZE MA, WANG PM, TEIXEIRA VP, REIS MA, PACHECO-SILVA A, CAMARA NO (2009) Early modulation of inflammation by mesenchymal stem cell after acute kidney injury. Int Immunopharmacol, 9: 677-682.
SHAKER OG, NASSAR YH, ASHOUR SS, HAMMAM OA (2015) Effect of mesenchymal stem cells on diabetic nephropathy in experimental animals. Med J Cairo Univ, 83(1): 1113-1122.
SRINIVASAN K, VISWANAD B, ASRAT L, KAUL CL, RAMARAO P (2005) Combination of high-fat diet-fed and low-dose streptozotocin-treated rat: a model for type 2 diabetes and pharmacological screening. Pharmacol Res, 52: 313-332.
SRIRAM PG, SUBRAMMANIAN S (2011) Fistin, a bioflavonoid ameliorates hyperglycemia in streptozotocin-induced experimental diabetes in rats. Int J Pharmac Sci Rev Res, 6 (1): 68-74.
TAKAHASHI T, HARRIS RC (2014) Role of endothelial nitric oxide synthase in diabetic nephropathy. lessons from diabetic eNOS knockout mice. J Diabetes Res, 2014: 590541.
VOLAREVIC V, NURKOVIC J, ARSENIJEVIC N, STOJKOVIC M (2014) Concise review: therapeutic potential of mesenchymal stem cells for the treatment of acute liver failure and cirrhosis. Stem Cells, 32: 2818-2823.
WANG SH, LI Y, ZHAO J, ZHANG J, HUANG Y (2013) Mesenchymal stem cells ameliorate podocyte injury and proteinuria in a type 1 diabetic nephropathy rat model. Biol Blood Marrow Transplant, 19: 538-546.
YANG Z, LI K, YAN X, DONG F, ZHAO C (2010) Amelioration of diabetic retinopathy by engrafted human adipose-derived mesenchymal stem cells in streptozotocin diabetic rats. Graefes Arch Clin Exp Ophthalmol, 248: 1415-1422.
ZHOU P, HOHM S, OLUSANYA Y, HESS DA, NOLTA JA (2009) Human progenitor cells with high aldehyde dehydrogenase. Hepatol, 49(6): 1992-2000.