Necrotizing enterocolitis (NE) is a medical condition mediated by oxidative stress to the small intestine. Fish oil is a natural component rich in healthy fatty acids such as eicosapentaenoic and docosahexaenoic acids with anti-inflammatory anti-apoptotic role. Endoplasmic reticulum stress mediates many oxidative diseases such as diabetes mellitus and neurodegeneration, associated by the release of many caspase subtypes that promotes cellular apoptosis. Mitochondrial stress also causes the release of many caspase variants together with mitochondrial Deoxyribonucleic acid (mtDNA). The aim of the present study is to appraise the role mediated by both endoplasmic reticulum (ER) &mitochondria stress in the pathogenesis of necrotizing enterocolitis and to explore the therapeutic effects of eicosapentaenoic and docosahexaenoic acids. Ninety rats’ pups were used (one day old), divided into three groups, (1) C-group: control group; (2) NE-group: necrotizing enterocolitis group, and (3) NE-FO-group: necrotizing enterocolitis pre-fed with fish oil group. At day 11, 12 and 13 ten pups from each group were sacrificed for intestinal sample collection. Histological examinations, Enzyme-linked immunosorbent assay, and western and Southern blot were performed. NE group showed severe histopathological changes. NE-FO group showed regain of most of normal histopathological feature. Levels of TNF-α and IL-6 increased markedly in NE group if compared to NE-FO group. Caspase 12 and caspase 9 levels in NE group were higher than inNE-FO group. The levels of GRB-78 and mtDNA were high in NE-FO group if compared to control group, but lower than levels of NE group. In conclusion, eicosapentaenoic acid and docosahexaenoic acid can protect against neonatal necrotizing enterocolitis. Mitochondrial and endoplasmic reticulum stress pathway demonstrated to mediate necrotizingenterocolitis pathological changes. Thus, fish oil offers a natural option to prevent and treat the clinical manifestation of NE.
Potential protective effect of eicosapentaenoic and docosahexaenoic acids versus necrotizing enterocolitis, mitochondrial and rough endoplasmic reticulum stress mediated pathway
Ahmed S. Ahmed
Anatomy and Embryology Department, College of Medicine, Tanta University. Tanta 31511, Egypt
SUMMARY
Sign up or Login
INTRODUCTION
Necrotizing enterocolitis (NE) is a medical emergency (Mehdi et al., 2012), first described in the eighteenth century and affecting mainly newborn infant of both sexes during the first four weeks of life. The underlying cause is poor blood supply to the intestine; resulting in tissue hypoxia, oxidative stress, inflammation [mediated by tumor necrosis factor-α (TNF-α) and interleukin-6(IL-6)] and cellular apoptosis (Li and Sheng, 2018). Drinking breast milk [rich in docosahexaenoic acid (DHA)] during the first days of life is considered prophylactic to the intestine from such pathology, as announced in 2012 by American Academy of Pediatrics (Pet et al., 2018). The typical clinical picture is feeding intolerance, abdominal distention and bloody stool. It may cause intestinal perforation and peritonitis. Many promising approaches for medical treatment appear in the horizon such as usage of DHA and arachidonic acid (Najm et al., 2017). Fish oil (FO) is extracted from fish, especially oily ones; it is marketed as a dietary supplement and ointments. Salmon-, sardine- and mackerel-extracted FO is the richest in content of healthy fatty acids (Serdarevic et al., 2019). To gain relative prophylaxis against prostate cancer, cardiovascular diseases, hypertension, Alzheimer disease and psoriasis, the daily dietary recommended intake of fish oil is 200 mg (Parker et al., 2019). FO was found to be very protective against many inflammatory bowel diseases such as Crohn’s disease (Parian and Limketkai, 2016), as it contains many anti-inflammatory compounds (Goel et al., 2018). It is very rich in omega-3 and omega-6 fatty acids, such as eicosapentaenoic acid (EPA) AND docosahexaenoic acid (DHA), which have anti-inflammatory role protecting the mucosal lining of the intestine (Zhang et al., 2018). These two fatty acids have an anti-apoptotic effect on hepatic, cardiac and nervous tissue cells through their rough endoplasmic reticulum and mitochondrial supporting role (Ajith, 2018).
Endoplasmic reticulum (ER) is a membranous cellular organelle which is classified as a cytoplasmic organelle (Phillips and Voeltz, 2016). Two types of ER are found, smooth (SER) and rough (RER). Both are formed of interconnected cisternae (Di, 2013), and they can be distinguished from each other by the ribosomes that are attached to the cytoplasmic surface of RER and absent from SER. RER is responsible for protein synthesis by transferring unfolded proteins to the Golgi apparatus, while SER is involved in lipid synthesis (Wang and Kaufman, 2016). Hypoxia, glucose deprivation and viral infection slow down the transfer of proteins from RER, which result in accumulation of unfolded proteins and glucose regulated protein-78 (GRP-78) in the cytoplasm of the cell, which is considered rough endoplasmic stress (RES) condition (Li et al., 2019). RES is the common share of many diseases such as diabetes mellitus, viral infection, neurodegeneration and neoplasm. RES is associated by the release of many caspase subtypes located on RER surface, causing cellular apoptosis (Lin et al., 2017).
Mitochondria is a double membrane enclosed organelle found in most of eukaryotic cells with some exceptions such as red blood cells (Nadalutti, 2020). It is responsible for adenosine triphosphate (ATP) synthesis (Hemono et al., 2020). It has an outer (smooth) membrane and inner (thrown into folds) membrane forming a shelf-like projection into the matrix of mitochondria called cristae. Obesity and excess oxidation results in mitochondrial stress with release of many caspase subtypes and mitochondrial Deoxyribonucleic acid (mtDNA) (Mohamed and Eltony, 2020; Lyu et al., 2020; Xie et al., 2020).
The aim of the present study is to appraise the role mediated by both RER and mitochondria stress in the pathogenesis of necrotizing enterocolitis and to explore the therapeutic effects of EPA and DHA.
MATERIALS AND METHODS
Chemicals
Fish oil, lipopolysaccharide water soluble (5 mg/ml), western and southern blot kits were purchased from Sino pharm Chemical Reagent Co., Ltd., Shanghai Shi, China.
Animals
Ninety albino Wistar rat pups were used (one day old), with an average weight of 20 gm. Animals were housed individually and fed with milk formula by gavage. 12-hour light/dark cycle was kept. By the help of air conditions, the temperature was kept at 25º C (in accordance to national and institutional guidelines), and humidity at 55%. This research study was approved by the Research and Ethics Committee, Quality Assurance Unit, Faculty of Medicine, Tanta University, Egypt.
Experimental design
Rats are divided into three groups (n=30). The control group (C-group) received saline by gavage for seven days (5ml/d); the necrotizing enterocolitis group (NE-group) received saline by gavage for seven days (5ml/d); and the necrotizing enterocolitis fed with fish oil group (NE-FO-group) received fish oil by gavage for seven days (5ml/d). Throughout the whole first ten days, the general appearance of abdominal size and stool was checked daily. NE rat model was performed along days 8, 9 and10 by creating as state of hypoxia and hypothermia to the pups, by placing them twice daily in hypoxic chamber for ninety seconds, and then Çransferring them to hypothermic chamber (4℃) for 600 seconds. Between the two settings, 10 mg/kg b.w. of lipopolysaccharides were administered by gavage to pups (Zhu et al., 2020). On day 11, 12 and 13, ten pups from each group were sacrificed for intestinal sample collection.
Clinical score
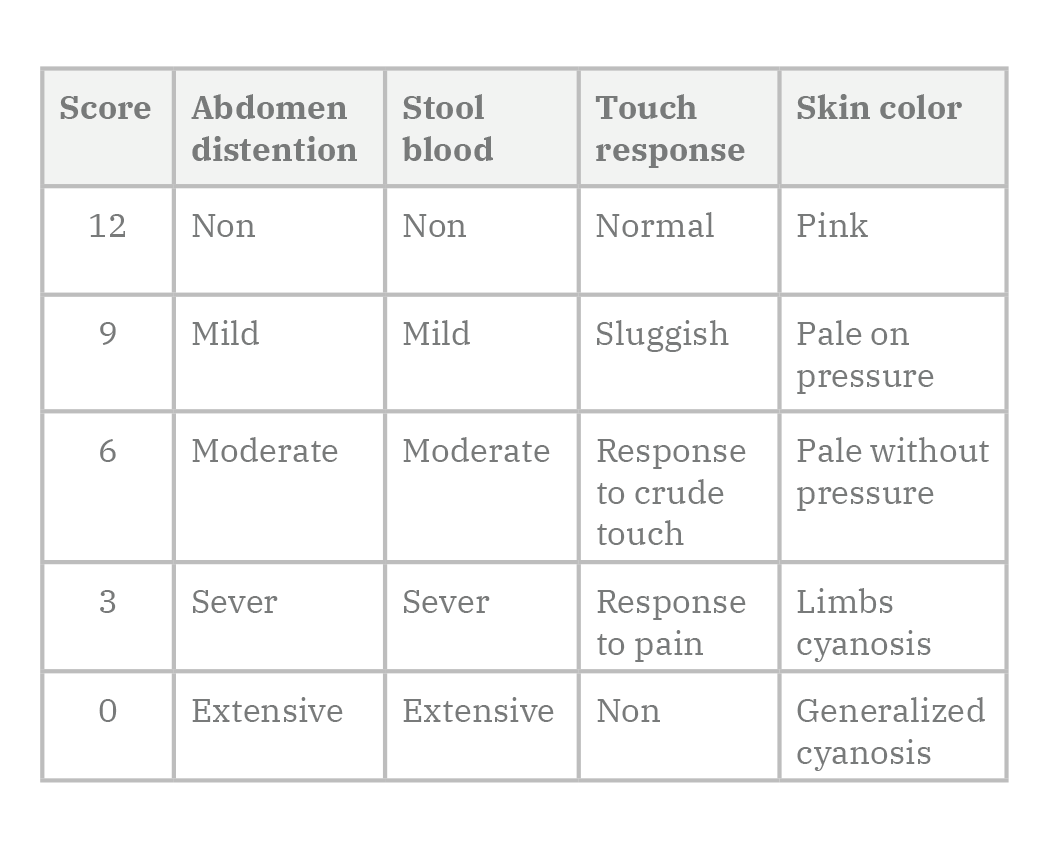
Pups body weight, abdominal distention, stool macroscopic blood, response to touch and color of skin were recorded daily and a score is given ranging from zero (worst) to twelve (best) (Table 1).
Histological examinations
Samples from the terminal part of the ileum were collected on days 11,12 and 13, and were fixed in 10% buffered paraformaldehyde, dehydrated, embedded in paraffin, and then sectioned at 5 μm by using 7manually operated rotary microtome CUT 4050 (4050F, R) (Microtec Laborgeräte GMBH, Germany). Sections were stained with hematoxylin and eosin for histopathological assessment. Histopathological examinations were performed by two expert histopathologists blinded to our study. Scoring was done as per lesion severity, as shown in (Table 2).
Enzyme-linked immunosorbent assay
Intestinal tissue was homogenized and centrifuged at 4000 rpm. Supernatant was used to detect TNF-α and IL-6 by help of the ELIZA kit (Sino pharm). Coating, blocking, incubation with enzyme-labelled antibody, substrate addition and signal detection are the main steps followed to detect the inflammatory markers (Kragstrup et al., 2013).
Western and southern blot
They are used to estimate the intestinal tissue content of caspase-9 and mtDNA as indicators for mitochondrial stress, and caspase-12 & GRB-78 as indicator of endoplasmic reticulum stress. Western blotting methodology started by extraction of proteins from intestinal tissues by the help of radioimmunoprecipitation assay (RIPA) buffer, followed by protein separation by gel electrophoresis, transfer to polyvinylidene fluoride (PVDF) membrane, blocking the rest of surface of high protein affinity membrane, incubation with primary antibody (caspase-12, caspase-9 & GRB-78) overnight, then incubation with secondary antibody for one hour finally analysis of the data expressed. Southern Blot kits were purchased from Thermo Fisher Scientific Inc. (USA). Anza™ T4 DNA Ligase Master Mix, IVGN2108 was used for mtDNA digestion. Fragmented DNA was typically electrophoresed on an Acrylamide gels; then DNA was transferred to a positively charged nylon membrane. Nucleic acid probe, RadPrime DNA Labeling System, 18428011 was incubated with the substrate, followed by hybridization and washing. BrightStar BioDetect Kit was used for mitochondrial DNA detection. (Dandelot and Gourdon, 2018).
Statistical analysis
Statistical Package for Social Sciences (SPSS) software, 20 V. (SPSS Inc., USA) was used for data analysis. The statistical significance of differences between groups was validated using one-way analysis of variance (ANOVA). Post hoc Tukey-Kramer test was used for group comparison. Data were expressed in mean ± standard deviation and probability value was considered significant if < 0.05.
RESULTS
Clinical score
The control group scored the best results after daily observation and scoring of each pup according to body weight, abdominal distention, stool macroscopic blood, response to touch and skin color. NE group showed significant (p < 0.05) score decrease, while NE-FO regained good results if compared to NE group, but its scoring was non-significantly (p > 0.05) lowered if compared to control group (Fig. 1).
Histological examinations
Histopathological examination of the intestinal wall showed normal histological architecture of intestinal wall layers in the control group, the four layers of intestinal wall (mucosa, submucosa, muscularis propria & adventitia) appeared healthy. NE group showed severe histopathological changes on day 13, mucosal inflammation, sloughing of villi, inflammatory cells infiltration and spacing between submucosa & muscularis mucosa were noticed. NE-FO group showed regain of most healthy histological feature if compared to control group, while some inflammatory cell infiltration was noticed on day 13 (Fig. 2).
Scoring of histopathological findings are shown in (Fig. 3). On day 11, 12 and 13, NE-FO group score was significantly (p < 0.05) lower than that of NE group, by 40.81%, 23.53%, and 34.34%, respectively.
Enzyme-linked immunosorbent assay
Levels of TNF-α and IL-6 increased significantly (p < 0.05) in NE group if compared to NE-FO group. Inflammatory markers levels in NE-FO group were significantly (p < 0.05) higher than that of the control group (Fig. 4). IL-6 scores of NE group on day 11,12 and13 were higher than those of control group by 8.08%, 61.74%, and 78.58%, respectively, and those of TNF-α in the NE group were higher than C group by 5.62%, 42.01%, and 72.82%, respectively.
Western and southern blot
Blotting of intestinal tissue proteins showed that caspase - 9, 12 levels in NE group were significantly (p < 0.05) increased if compared to NE-FO group. The levels of GRB-78 & mtDNA were significantly (p < 0.05) higher if NE-FO group was compared to control group, but significantly (p < 0.05) lower than that of NE group (Fig. 5).
DISCUSSION
Necrotizing enterocolitis is a medical problem that came to the surface along the past 30 years. 90% of NE patients are premature infants with 20% mortality rate. This research paper was designed to understand the underlying mechanism NE on a cellular basis and to explore the protective effects of some omega-3 fatty acids.
Our results showed that there is a marked decrease in NE clinical score if compared to the control group, which comes in agreement with other researchers. Aktaş et al. (2017) reported massive fecal blood as a common sign of NE, mainly in premature infants. Abdominal distention and absence of intestinal sound was reported by Lin et al. (2019). We found a decrease in response to touch with peripheral cyanosis in NE group, and to a less extent in NE-FO group in certain parameters such as abdominal distention. The clinical score of NE-FO was enhanced due to the effect of the ingested fish oil as agreed by Najm et al. (2017), who reported the beneficial effects of fish oil on necrotizing enterocolitis, bronchopulmonary dysplasia and patency of ductus arteriosus.
Histopathological examination of terminal part of the ileum in the control group showed healthy layers of intestinal wall with vivid villi, submucosa, muscle layer and the outer serosa covering (Inamoto et al., 2008). In NE group, our results showed a severe intestinal wall inflammation with inflammatory cells infiltration (mainly mucosal lining) with necrosis and sloughing of villi. Edema and spacing appeared between mucosa and submucosa, which comes in agreement with Gross et al. (2017), who added that inhibition of 5-hydroxytryptamine synthesis reduced the mucosal inflammation on intestine. McElroy et al. (2013) hypothesized that blood vessels vasculitis and occlusion may be the direct cause of villous necrosis in NE. While, MohanKumar et al. (2019) reported that submucosal edema occurs within twelve hours of NE onset associated with sever mucosal inflammation and villous necrosis.
In the present study, the intestinal histological architecture in NE-FO group appeared to be healthy, with regain of most of normal histopathological feature after fish oil pretreatment: this could be explained by its content of both docosahexaenoic and eicosapentaenoic acids with their anti-inflammatory and antioxidant potential. Molfino et al. (2017) reported that DHA has the ability to inhibit different inflammatory pathways by inhibiting inflammatory-related cytokines synthesis. This anti-inflammatory potential was reported by Irún et al. (2019), who reported that the anti-inflammatory potential of DHA is not associated with immunosuppression. Ochi et al. (2018) reported the antioxidant and anti-inflammatory capacity of EPA mainly in muscle tissue after exercise related stress.
Our study showed that Levels of TNF-α and IL-6 increased markedly in NE group if compared to NE-FO group. This comes in concession with Li et al. (2018), who reported that inflammatory markers reach their peak within twenty-four hours and start to fall after seventy-two hours. This could be due to the anti-inflammatory capacity of eicosapentaenoic acid as stated by Augimeri et al. (2019).
However, Gomes et al. (2017) mentioned that NE is associated with elevated levels of caspase-3 which mediate cellular apoptosis. In addition, Buyuktiryaki et al. (2019) reported elevated levels of caspase 3, 8 and 9 in NE. In our study, NE group showed elevated caspase 9 and caspase 12 levels in intestinal tissue higher than those of NE-FO group. Katoh et al. (2004) mentioned that caspase-9 accumulate in the stressed mitochondria. Other researcher stated that caspase-12 is elevated in ischemic brain tissue after ischemia-reperfusion model as a sign of endoplasmic reticulum stress (Xing et al., 2019). The relative decrease in apoptotic markers noticed at our research in NE-FO group could be related to the anti-apoptotic effect of EPA. Fayez and Zaafan (2018) reported the potent antiapoptotic, anti-inflammatory and antioxidant capacity of EPA.
In our study, levels of GRB-78 and mtDNA were high in NE-FO group if compared to control group, but lower than levels of NE group which could result from endoplasmic reticulum and mitochondrial stress respectively. Zhu et al. (2020) linked between ER stress and elevated tissue level of GRP-78. Ding et al. (2011) mentioned also the elevated level of GRB-78 in the kidney tissue resulting from ER stress. Baregamian et al. (2009) stated that necrotizing enterocolitis is associated with disturbed mitochondrial functions and elevated levels of mtDNA in intestinal tissue. Other researcher (Sas et al., 2018) related healthy mtDNA to properly functioning mitochondria. Shao et al. (2006) reported the upregulated mtDNA in cases of mitochondrial stress. The decreased levels of GRB-78 and mtDNA after fish oil pretreatment in NE-FO group may be due to the anti-apoptotic effect of DHA, as stated by Bazan et al. (2010). In conclusion, the present study revealed the shielding effect of eicosapentaenoic acid and docosahexaenoic acid against neonatal necrotizing enterocolitis through their anti- inflammatory and antioxidant effects. We also demonstrated that necrotizing enterocolitis is mediated by mitochondrial and endoplasmic reticulum stress pathway. So, fish oil offers a natural option to prevent and treat the clinical manifestation of NE. Further studies are requires determining the exact signaling pathway mediating NE pathological changes.
Related articles
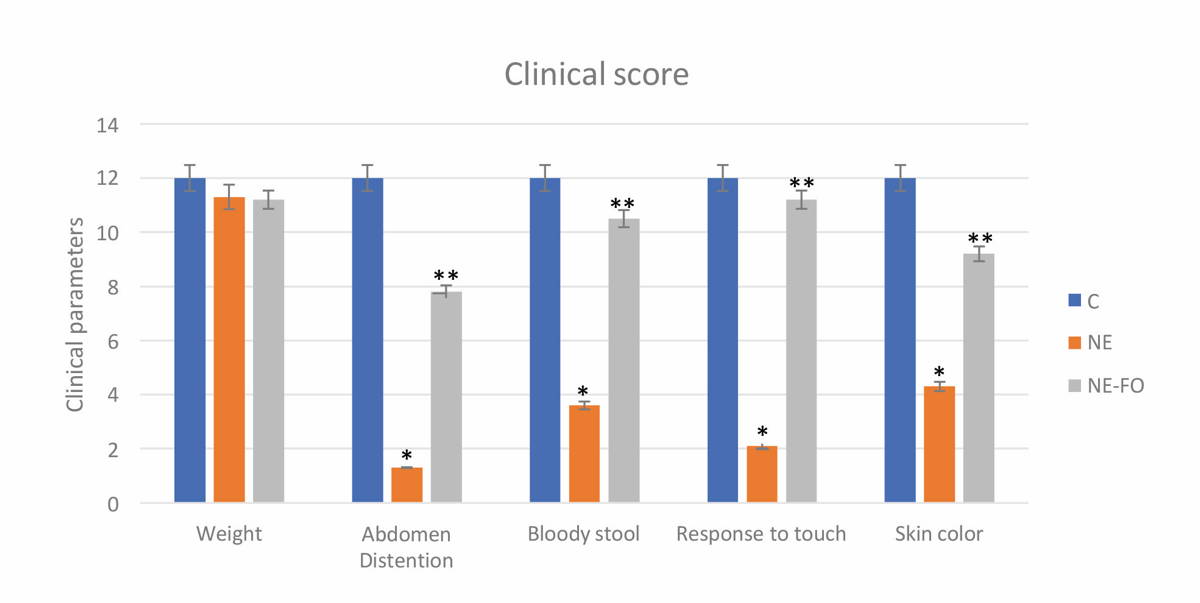 Fig. 1.- Clinical score represents changes in body weight, abdominal distention, stool macroscopic blood, response to touch and color of skin. (C) control group, (NE) necrotizing enterocolitis group, (NE-FO) necrotizing enterocolitis fed with fish oil group. * significant difference (p < 0.05) in comparison to C-group. ** significant difference (p < 0.05) in comparison with NE-group. Data are presented as mean ± standard deviation, (n = 30).
Fig. 1.- Clinical score represents changes in body weight, abdominal distention, stool macroscopic blood, response to touch and color of skin. (C) control group, (NE) necrotizing enterocolitis group, (NE-FO) necrotizing enterocolitis fed with fish oil group. * significant difference (p < 0.05) in comparison to C-group. ** significant difference (p < 0.05) in comparison with NE-group. Data are presented as mean ± standard deviation, (n = 30).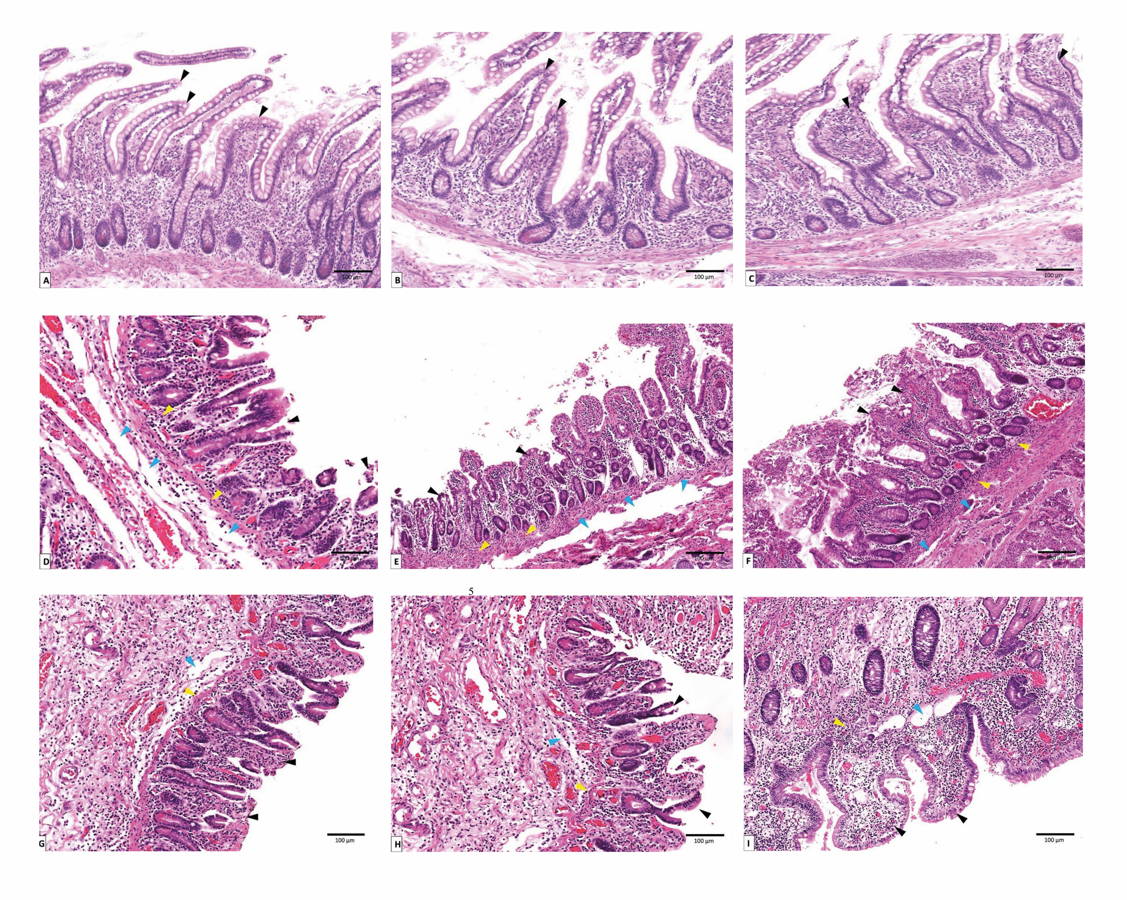 Fig. 2.- Photomicrograph of sections of rats’ terminal part of the ilium stained with hematoxylin and eosin, magnification (x 1000). (A, B, C) represent control group on days 11, 12 and 13 respectively. (D, E, F) represent NE group on days 11, 12 and 13 respectively. (G, H, I) represent NE-FO group on days 11, 12 and 13 respectively. Note: villi (black arrow), submucosal edema (blue arrow), inflammatory cells infiltration (yellow arrow).
Fig. 2.- Photomicrograph of sections of rats’ terminal part of the ilium stained with hematoxylin and eosin, magnification (x 1000). (A, B, C) represent control group on days 11, 12 and 13 respectively. (D, E, F) represent NE group on days 11, 12 and 13 respectively. (G, H, I) represent NE-FO group on days 11, 12 and 13 respectively. Note: villi (black arrow), submucosal edema (blue arrow), inflammatory cells infiltration (yellow arrow).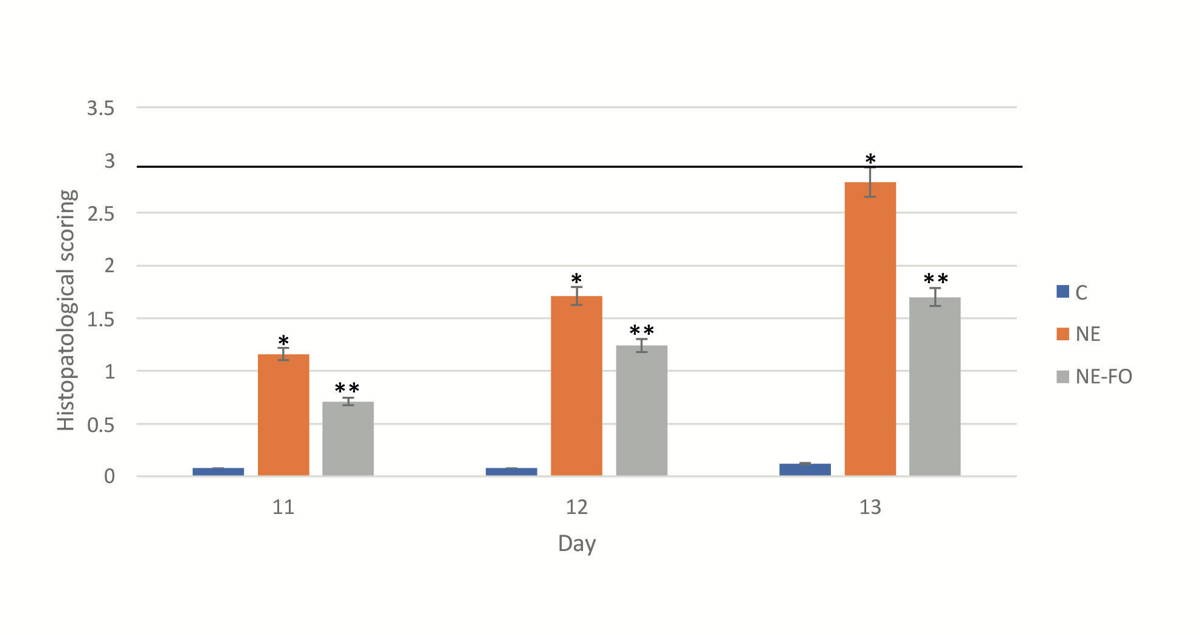 Fig. 3.- Scoring of histopathological finding done by blinded observer to our study. * significant difference (p < 0.05) in comparison to C-group. ** significant difference (p < 0.05) in comparison with NE-group. Data are presented as mean ± standard deviation, (n = 30).
Fig. 3.- Scoring of histopathological finding done by blinded observer to our study. * significant difference (p < 0.05) in comparison to C-group. ** significant difference (p < 0.05) in comparison with NE-group. Data are presented as mean ± standard deviation, (n = 30).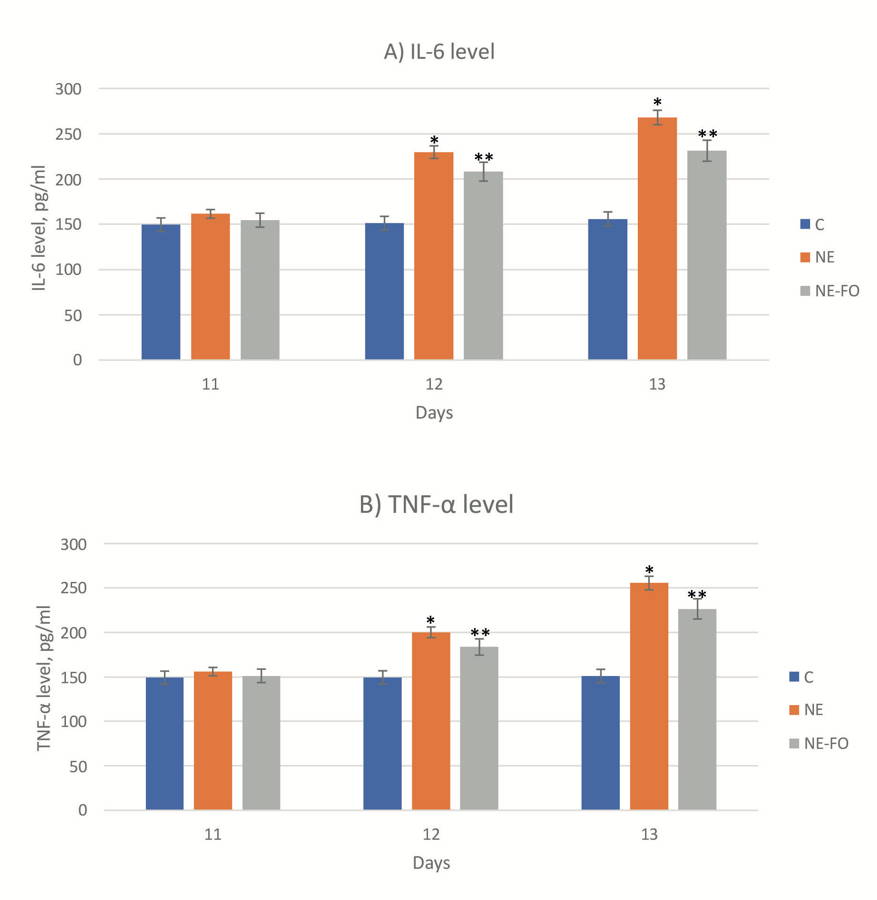 Fig. 4.- Level of inflammatory markers in the intestinal tissueon days 11, 12 and 13 (A) IL-6 level. (B) TNF-α level. * significant difference (p < 0.05) in comparison to C-group. ** significant difference (p < 0.05) in comparison with NE-group. Data are presented as mean ± standard deviation, (n = 30).
Fig. 4.- Level of inflammatory markers in the intestinal tissueon days 11, 12 and 13 (A) IL-6 level. (B) TNF-α level. * significant difference (p < 0.05) in comparison to C-group. ** significant difference (p < 0.05) in comparison with NE-group. Data are presented as mean ± standard deviation, (n = 30).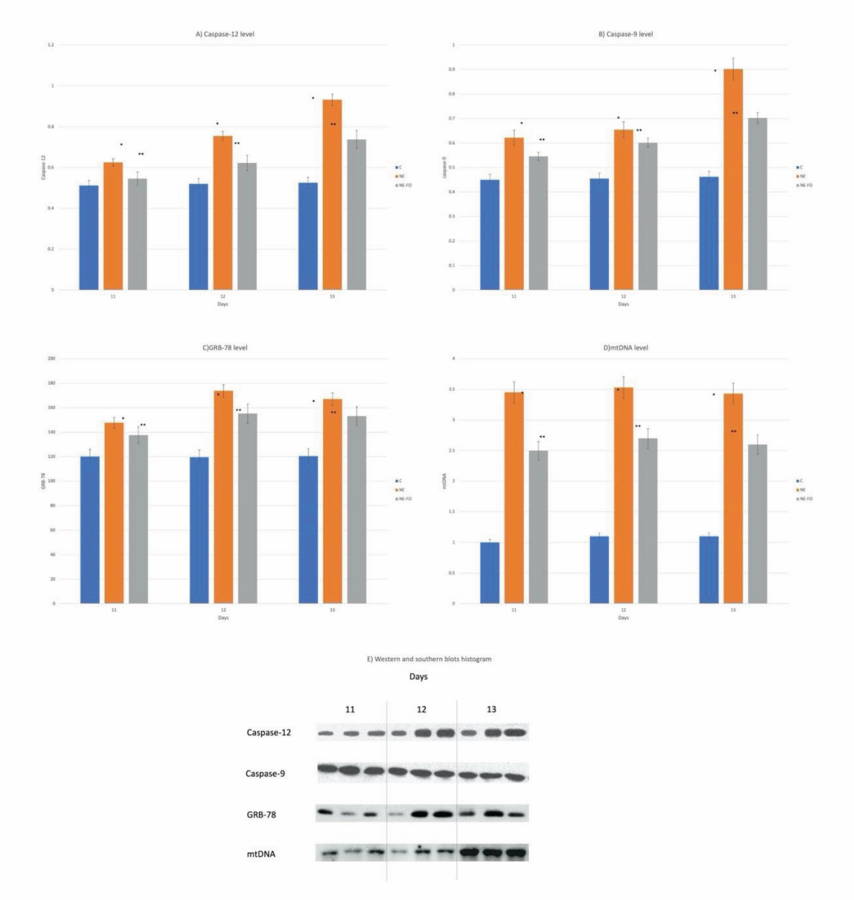 Fig. 5.- (A, B and C) represent western blot quantitative analysis of caspase 12, caspase 9 and GRB-78, respectively. (D) represents southern blot quantitative analysis of mtDNA in the intestinal tissue (days 11, 12 and 13). (E) Histogram represents the ratio of optical density of caspase-12, caspase-9, GRB-78 and mtDNA. * significant difference (p < 0.05) in comparison to C-group. ** significant difference (p < 0.05) in comparison with NE-group. Data are presented as mean ± standard deviation, (n = 30).
Fig. 5.- (A, B and C) represent western blot quantitative analysis of caspase 12, caspase 9 and GRB-78, respectively. (D) represents southern blot quantitative analysis of mtDNA in the intestinal tissue (days 11, 12 and 13). (E) Histogram represents the ratio of optical density of caspase-12, caspase-9, GRB-78 and mtDNA. * significant difference (p < 0.05) in comparison to C-group. ** significant difference (p < 0.05) in comparison with NE-group. Data are presented as mean ± standard deviation, (n = 30).AJITH TA (2018) A recent update on the effects of omega-3 fatty acids in Alzheimer's disease. Curr Clin Pharmacol, 13(4): 252-260.
AKTAŞ S, ERGENEKON E, ÜNAL S, TÜRKYILMAZ C, HIRFANOĞLU İM, ATALAY Y (2017) Different presentations of cow`s milk protein allergy during neonatal period. Turk J Pediatr, 59(3): 322-328.
AUGIMERI G, PLASTINA P, GIONFRIDDO G (2019) N-Eicosapentaenoyl dopamine, a conjugate of dopamine and eicosapentaenoic acid (EPA), exerts anti-inflammatory properties in mouse and human macrophages. Nutrients, 11(9): 2247.
BAREGAMIAN N, SONG J, BAILEY CE, PAPACONSTANTINOU J, EVERS BM, CHUNG DH (2009) Tumor necrosis factor-alpha and apoptosis signal-regulating kinase 1 control reactive oxygen species release, mitochondrial autophagy, and c-Jun N-terminal kinase/p38 phosphorylation during necrotizing enterocolitis. Oxid Med Cell Longev, 2(5): 297-306.
BAZAN NG, CALANDRIA JM, SERHAN CN (2010) Rescue and repair during photoreceptor cell renewal mediated by docosahexaenoic acid-derived neuroprotectin D1. J Lipid Res, 51(8): 2018-2031.
BUYUKTIRYAKI M, TAYMAN C, KOYUNCU I (2019) Therapeutic and preventative effects of ankaferd blood stopper in an experimental necrotizing enterocolitis model. Biomed Pharmacother, 110: 105-110.
DANDELOT E, GOURDON G (2018) The flash-small-pool PCR: how to transform blotting and numerous hybridization steps into a simple denatured PCR. Biotechniques, 64 (6): 262-265.
DI GIROLAMO M, FABRIZIO G, SCARPA ES, DI PAOLA S (2013) NAD⁺-dependent enzymes at the endoplasmic reticulum. Curr Top Med Chem, 13(23): 3001-3010.
DING Y, ZOU J, LI Z (2011) Study of histopathological and molecular changes of rat kidney under simulated weightlessness and resistance training protective effect. PLoS One, 6(5): e20008.
FAYEZ AM, ZAAFAN MA (2018) Eicosapentaenoic acid and vitamin E against doxorubicin induced cardiac and renal damages: role of cytochrome c and iNOS. Arch Iran Med, 21(11): 502-508.
GOEL A, POTHINENI NV, SINGHAL M, PAYDAK H, SALDEEN T, MEHTA JL (2018) Fish, fish oils and cardioprotection: promise or fish tale? Int J Mol Sci, 19(12): 3703.
GOMES RO, ARTIGIANI R NETO, GUIMARÃES JF NETO, NUNES AP, MONTERO EF, MARTINS JL (2017) Neonatal necrotizing enterocolitis rat model attenuated by a remote ischemic preconditioning in the pregnant. Acta Cir Bras, 32(3): 236-242.
GROSS MARGOLIS K, VITTORIO J, TALAVERA M (2017) Enteric serotonin and oxytocin: endogenous regulation of severity in a murine model of necrotizing enterocolitis. Am J Physiol Gastrointest Liver Physiol, 313(5): G386-G398.
HEMONO M, UBRIG É, AZEREDO K, SALINAS-GIEGÉ T, DROUARD L, DUCHÊNE AM (2020) Arabidopsis voltage-dependent anion channels (VDACs): overlapping and specific functions in mitochondria. Cells, 9(4): E1023.
INAMOTO T, KAWATA Y, QI WM (2008) Ultrastructural study on the epithelial responses against attachment of indigenous bacteria to epithelial membranes in Peyer's patches of rat small intestine. J Vet Med Sci, 70(3): 235-241.
IRÚN P, LANAS A, PIAZUELO E (2019) Omega-3 polyunsaturated fatty acids and their bioactive metabolites in gastrointestinal malignancies related to unresolved inflammation. a review. Front Pharmacol, 10: 852.
KATOH I, TOMIMORI Y, IKAWA Y, KURATA S (2004) Dimerization and processing of procaspase-9 by redox stress in mitochondria. J Biol Chem, 279(15): 15515-15523.
KRAGSTRUP TW, VORUP-JENSEN T, DELEURAN B, HVID M (2013) A simple set of validation steps identifies and removes false results in a sandwich enzyme-linked immunosorbent assay caused by anti-animal IgG antibodies in plasma from arthritis patients. Springer plus, 2(1): 263.
LI J, LI X, LIU D (2019) EIF2α signaling regulates autophagy of osteoblasts and the development of osteoclasts in OVX mice. Cell Death Dis, 10(12): 921.
LI Z, SHENG L (2018) Significance of dynamic evolution of TNF-α, IL-6 and intestinal fatty acid-binding protein levels in neonatal necrotizing enterocolitis. Exp Ther Med, 15(2): 1289-1292.
LIN L, XIA X, LIU W, WANG Y, HUA Z (2019) Clinical characteristics of neonatal fulminant necrotizing enterocolitis in a tertiary Children's hospital in the last 10 years. PLoS One, 14(11): e0224880.
LIN Y, HUANG JJ, DAHMS HU, ZHEN JJ, YING XP (2017) Cell damage and apoptosis in the hepatopancreas of Eriocheir sinensis induced by cadmium. Aquat Toxicol, 190: 190-198.
LYU AR, KIM TH, PARK SJ (2020) Mitochondrial damage and necroptosis in aging cochlea. Int J Mol Sci, 21(7): E2505.
MCELROY SJ, UNDERWOOD MA, SHERMAN MP (2013) Paneth cells and necrotizing enterocolitis: a novel hypothesis for disease pathogenesis. Neonatology, 103(1): 10-20.
MEHDI I, AL BAHRANI B (2012) Chemotherapy-induced neutropenic necrotizing enterocolitis: a review. J Pak Med Assoc, 62(7): 718-723.
Mohamed HK, Eltony SA (2020) Effect of acute pentylenetetrazol injection induced epileptic seizures on rat dentate gyrus at different postnatal ages. Anat Cell Biol, 53(1): 84-94.
MOHANKUMAR K, NAMACHIVAYAM K, SONG T (2019) A murine neonatal model of necrotizing enterocolitis caused by anemia and red blood cell transfusions. Nat Commun, 10(1): 3494.
MOLFINO A, AMABILE MI, MONTI M, MUSCARITOLI M (2017) Omega-3 polyunsaturated fatty acids in critical illness: anti-inflammatory, proresolving, or both? Oxid Med Cell Longev, 2017: 5987082.
NADALUTTI CA, STEFANICK DF, ZHAO ML (2020) Mitochondrial dysfunction and DNA damage accompany enhanced levels of formaldehyde in cultured primary human fibroblasts. Sci Rep, 10(1): 5575.
NAJM S, LÖFQVIST C, HELLGREN G (2017) Effects of a lipid emulsion containing fish oil on polyunsaturated fatty acid profiles, growth and morbidities in extremely premature infants: A randomized controlled trial. Clin Nutr ESPEN, 20: 17-23.
OCHI E, TSUCHIYA Y (2018) Eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) in muscle damage and function. Nutrients, 10(5): 552.
PARIAN A, LIMKETKAI BN (2016) Dietary supplement therapies for inflammatory Bowel disease: Crohn's disease and ulcerative colitis. Curr Pharm Des, 22(2): 180-188.
PARKER HM, COHN JS, O'CONNOR HT (2019) Effect of fish oil supplementation on hepatic and visceral fat in overweight men: a randomized controlled trial. Nutrients, 11(2): 475.
PET GC, MCADAMS RM, MELZER L (2018) Attitudes surrounding the management of neonates with severe necrotizing enterocolitis. J Pediatr, 199: 186-193, e3.
PHILLIPS MJ, VOELTZ GK (2016) Structure and function of ER membrane contact sites with other organelles. Nat Rev Mol Cell Biol, 17(2): 69-82.
SAS K, SZABÓ E, VÉCSEI L (2018) Mitochondria, oxidative stress and the kynurenine system, with a focus on ageing and neuroprotection. Molecules, 23(1): 191.
SERDAREVIC N, PASALIC A, DJIDO V, PECAR M, TRTAK N, GOJAK R (2019) The vitamine source, usual food intake at students. Mater Sociomed, 31(1): 53-56.
SHAO H, LAN D, DUAN Z (2006) Upregulation of mitochondrial gene expression in PBMC from convalescent SARS patients. J Clin Immunol, 26(6): 546-554.
WANG M, KAUFMAN RJ (2016) Protein misfolding in the endoplasmic reticulum as a conduit to human disease. Nature, 529 (7586): 326-335.
XIE W, JIAO B, BAI Q (2020) Chemoptogenetic ablation of neuronal mitochondria in vivo with spatiotemporal precision and controllable severity. Elife, 9: e51845.
XING J, XU H, LIU C (2019) Melatonin ameliorates endoplasmic reticulum stress in N2a neuroblastoma cell hypoxia-reoxygenation injury by activating the AMPK-Pak2 pathway. Cell Stress Chaperones, 24(3): 621-633.
ZHANG T, WANG N, YAN W (2018) Effect of a fish oil-based lipid emulsion on intestinal failure-associated liver disease in children. Eur J Clin Nutr, 72(10): 1364-1372.
ZHU X, CUI N, YU L (2020) Potential role of endoplasmic reticulum stress is involved in the protection of fish oil on neonatal rats with necrotizing enterocolitis. Sci Rep, 10 (1): 6448.