The dentate gyrus is a sensitive region of the brain for learning and memory, and is thus vulnerable to post-ischemic changes. So, this work aimed to study the histological changes in the dentate gyrus after right common carotid artery occlusion/reperfusion. To the best of our knowledge, this research is the first attempt to demonstrate the beneficial protective role of red beetroot supplementation. Forty-two adult male albino rats were divided randomly into: Group I, consisting of 18 rats and were subdivided into control, red beetroot extract (RBE), and sham; Group II, right common carotid artery occlusion (RCCAO) group (n = 12). This group had unilateral common carotid artery occlusion on the right side for 90 minutes followed by reperfusion for one week. Group III: RCCAO+RBE group (n = 12): received RBE (500 mg/kg/day/oral) for 22 days, and on day 15 were subjected to RCCAO then reperfusion for one week. At the end of the experiment, the dentate gyrus sections were subjected to histological, immunohistochemical, and morphometric analyses for the expression of Caspase-3, glial fibrillary acidic protein (GFAP), Heat shock protein 70 (HSP70), Tumor necrosis factor-alpha (TNF-α). Besides, tissue nitric oxide, superoxide dismutase, and malonaldehyde levels were measured. Results: RBE ameliorates the ischemic induced dentate gyrus neurodegenerative changes. The number of granular cells and their Nissl’s granules content were significantly increased. This study clarified that red beetroot extract exerted its effects via regulating oxidative stress, inflammation, and apoptosis. Besides, this study detected that the beneficial effect of RBE was also mediated by modulating HSP70 expression and the astroglial response to the injury. Conclusion: Red beetroot extract showed an anti-ischemic potential providing new insight into its significance as a neuroprotective agent.
Influence of global cerebral ischemia/reperfusion injury on rat dentate gyrus and the possible protective effect of beetroot (Beta vulgaris L.) extract
Samah Elsayed1, Mostafa El-Habeby1, Neveen El-Sherif1, Marwa Al-Gholam1
Department of Anatomy and Embryology, Faculty of Medicine, Menoufia University, Menoufia, Egypt
SUMMARY
Sign up or Login
INTRODUCTION
According to the World Health Organization, 15 million people suffer strokes worldwide each year (Virani et al., 2020). Of these, 5 million die, and another 5 million are permanently disabled (Mozaffarian et al., 2016). Stroke is the second cause of death after ischemic heart disease, and over a third of deaths by stroke occur in developing countries. Arab countries constitute populations with a similar lifestyle and diet that may influence stroke risk, type, and survival after stroke (Benamer and Grosset, 2009). The incidence of stroke in low-income and middle-income countries exceeded that in high-income countries by 20% (Lee et al., 2015). In Egypt, according to recent estimates, the overall prevalence rate of stroke is 963/100,000 inhabitants (Abd-Allah and Moustafa, 2014).
The main symptoms involve impairments in vision, body movement, and speaking. It can include unconsciousness, blindness, problems with coordination, and weakness in the body. Other effects that may result from brain ischemia are stroke, cardiorespiratory arrest and irreversible brain damage (Lozano et al. ,2013). Oxidative stress, leukocyte infiltration, platelet activation and aggregation, complement activation, and breakdown of the blood-brain barrier (BBB) are the major mechanisms of reperfusion injury. These mechanisms ultimately lead to edema or hemorrhagic transformation (Kim and Johnston, 2011). Despite stroke being a serious life-threatening risk and causing disability, the only effective accepted treatment is tissue plasminogen activators. However, the duration limitations of this treatment mean that it can only be given to a small proportion of patients, 1-2% (Goldstein, 2007).
Despite decades of intense research, the beneficial treatment of stroke remains limited (Lozano et al., 2013). In light of this, the search for effective means ameliorating cerebral ischemia-reperfusion injury (CIRI) is one of the major problems of experimental medicine and biology (Goldstein, 2007).
The powerful antioxidant, anti-inflammatory, and vascular-protective effects offered by beetroot and its constituents have been proved by several in vitro and in vivo human and animal studies (Clifford et al., 2015).
Consequently, the present study was designed to investigate, for the first time to the best of our knowledge, the protective effect of red beetroot extract on the post-ischemic dentate gyrus neurodegeneration.
MATERIALS AND METHODS
Chemicals
Red Beetroots (Beta vulgaris) were purchased from a local market. One kilogram of Fresh Red Beetroots was exhaustively macerated by soaking in 70% (1.5 L) ethanol for three successive days after washing with tap water and cutting into small pieces. Then this alcoholic extract was concentrated under reduced pressure using a rotatory evaporator till complete drying (Biochemistry Department, Faculty of Science, Menoufia University, Egypt) (El Gamal et al., 2014). The resulted extract (RBE, 150 g) was later suspended in distilled water.
Animals
Forty-two adult male albino rats with an average weight of 200-250 grams were used in this study. They were obtained from Ain shams Animal House, Cairo, Egypt. The animals were kept under controlled conditions of temperature and humidity and provided with water and a balanced diet. The light / darkness cycle was fixed at 12:12 h. The procedure was approved by the ethics committee on the animal experiment of the Faculty of Medicine, Menoufia University, Egypt following the international regulations on care and use of laboratory animals.
The rats were randomly divided into 3 groups:
Group I consisted of 18 rats and were subdivided into 3 subgroups. Subgroup Ia (n=6): animals were fed a regular diet. Subgroup Ib (n = 6): the rats were subjected to a sham surgical procedure on day 15 and left without treatment. Subgroup Ic (n=6): the rats received red beetroot extract (RBE) (500 mg/kg/day/orally) (El Gamal et al., 2014) for 22 days.
Group II: (RCCAO) group (n = 12). This group had unilateral common carotid artery occlusion on the right side for 90 minutes (Mentari et al.,2018) followed by reperfusion for one week.
Group III: (RCCAO+RBE) protected group (n = 12). The rats received red beetroot extract (RBE) (500 mg/kg/day/orally) (El Gamal et al., 2014) for 22 days and were subjected to the same surgical procedure as the group II followed by reperfusion for one week.
Surgical procedure
Induction of cerebral ischemia/reperfusion was carried out using the standard method (Mentari et al., 2018). Overnight fasted rats were anesthetized with thiopental sodium (30 mg/kg). A midline ventral incision was made in the throat. The right common carotid artery was located and freed from the surrounding tissue and vagus nerve. Global cerebral ischemia was induced by occluding the carotid artery on the right side with a clamp. After 90 min of global cerebral ischemia, the clamp was removed to allow the reperfusion of blood through the carotid artery. Rats were maintained at 37°C on a heated surgical platform. All surgical procedures were carried out under sterile conditions. The surgical procedure was done at Ain Shams University, Faculty of Medicine, Egypt.
At the end of the experiment, rats were deeply anesthetized using ketamine (90 mg/kg) and xylazine (15 mg/kg) intraperitoneally, then decapitated. The dentate gyrus sections were subjected to biochemical, histological, immunohistochemical, and morphometric analyses.
Evaluation methods
Histological study
A coronal section was performed in the cerebral hemisphere into two halves to reach the site of the dentate gyrus and then fixed in 10% buffered formaldehyde solution; then the specimens were dehydrated, cleared, and embedded in paraffin blocks. Serial coronal sections were cut 5 μm thick and stained with hematoxylin and eosin (H & E) for routine histological examination and with toluidine blue (TB) to detect Nissl’s granules.
Immunohistochemical study
The paraffin sections on poly-L-lysin-coated slides were deparaffinized and rehydrated. Endogenous peroxidase was blocked by inserting the sections in 3% hydrogen peroxide (H2O2). The microwave antigen retrieval procedure was performed. The sections were incubated with primary anti Heat shock protein 70 (HSP70 antibody); a marker for oxidative stress- cytoplasmic and nuclear expression (rabbit polyclonal antibody, Midco Trade Company, Giza, Egypt); anti-Caspase-3 antibody; an apoptotic marker cytoplasmic expression, (rabbit polyclonal antibody, Dako, Carpinteria California, USA); anti-Glial fibrillary acidic protein (GFAP) antibody; a marker for astrocyte activation (rabbit polyclonal antibody, Midco Trade Company, Giza, Egypt) and anti-Tumor necrosis factor-alpha (TNF-α) antibody, expressed in the cytoplasm of granular cells (mouse monoclonal antibody, Gene tex company, Cairo, Egypt). After that, the biotinylated goat-polyvalent secondary antibody was applied. The sections were then incubated in preformed streptavidin-peroxidase and, finally, we applied the prepared DAB substrate chromogen (3,3′-diaminobenzidine tetrahydrochloride). The slides were counterstained with hematoxylin to be examined under a light microscope.
Biochemical study
Preparation of brain tissue for estimation of biochemical parameters related to oxidative stress. The brain of each animal was removed and washed with cooled 0.9% saline, kept on ice, and subsequently blotted on filter paper, then weighed and homogenized with cold phosphate buffer (0.1M, pH 7.4) using a Remi homogenizer. The homogenization procedure was performed as quickly as possible under completely standardized conditions. The homogenate was centrifuged at 1,000 rpm and 4°C for 3 min, and the supernatant was divided into two portions, one of which was used for measurement of Malondialdehyde (MDA). The remaining supernatant was again centrifuged at 12,000 rpm at 4°C for 15 min and used for the measurement of superoxide dismutase (SOD) levels by standard methods (PanelKevin et al., 1985).
Morphometric assessment
For histological and immunohistochemical quantitative assessment, from each section six non-overlapping fields (400x) were randomly captured by a Leica Microscope DML B2/11888111 equipped with a Leica camera DFC450. The different parameters including the thickness of the granular layer, number of granular cells, the color intensity of toluidine blue, number of GFAP positive cells, Caspase-3 positive cells and HSP70 positive cells, and the area percentage of TNF-α immunoreaction were assessed in the fields taken from at least six sections/animal using image J software (Maryland, USA) and averaged per field for each animal. The calculated data for at least six animals/experimental group and the biochemical results were subjected to statistical analysis.
Statistical analysis
Mean ± SD was used to present the collected data. Data analysis was carried out using SPSS (Inc., Chicago, IL, USA) version 23 on IBM compatible computer. The obtained data were analyzed using one way-ANOVA followed by the post hoc Bonferroni test. The results were considered statistically significant when the p-values were <0.05.
RESULTS
Examination of all subgroups in group I showed the same histological findings and revealed no statistically significant difference in all the examined parameters between them. So we considered all as a control group.
Histological results
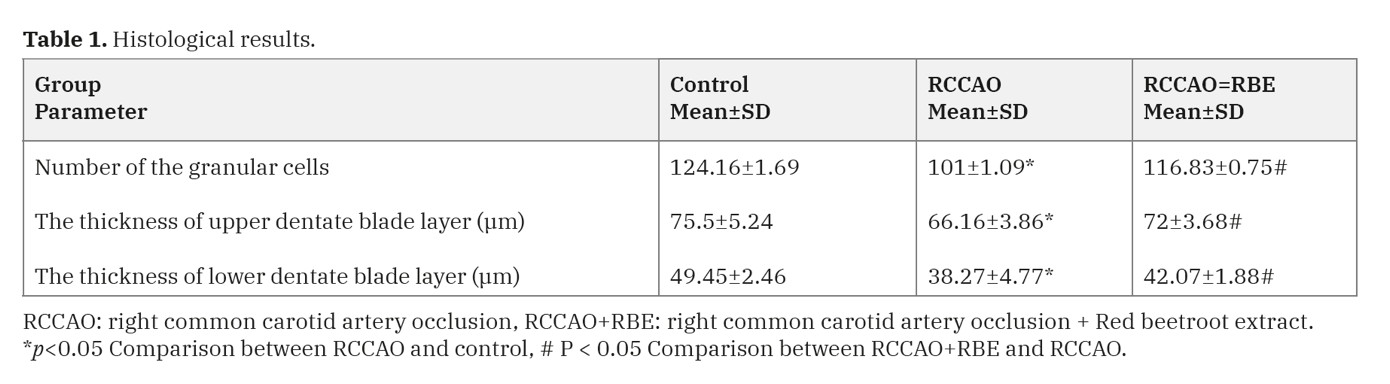
In H&E-stained sections, the hippocampus appears as a pair of interlocking C-shaped structures: Ammon’s horn and the dentate gyrus. The dentate gyrus of the control group was formed of molecular, granular, and polymorphic layers (Fig. 1a). The granular layer was formed of multiple regular rows of well-organized, compactly arranged granular cells that were arranged in a V-shaped configuration forming upper and lower blades. Each granular cell appeared elliptical with basophilic cytoplasm, large vesicular nucleus, and prominent nucleolus. Both molecular and polymorphic layers were formed mainly of the eosinophilic neuropil matrix within which neuroglia was embedded. Microglia with rod-shaped nuclei, oligodendroglia with small dark nuclei and perinuclear halos, and astrocytes with oval vesicular nuclei were observed (Fig. 1b). In the RCCAO group, there was a disturbing arrangement of the granular cells that appeared degenerated with shrunken hyperchromatic nuclei, perinuclear halos, and lost nuclear details. The neuropil showed many vacuolations and congested blood vessels. Moreover, the number of astrocytes was increased compared with the control group (Fig. 1c). In the RCCAO+RBE group, many normal granular cells were observed with only some pyknotic ones and few ghost cells. The neuropil was regularly organized except for the presence of few vacuolations and less congested blood vessels. Besides, few astrocytes were observed. Few spindle-shaped cells were detected in SGZ. (Fig. 1d). Statistically, the thickness of the granular blades was significantly decreased (P<0.05) in the RCCAO group compared with the control one. However, it showed a significant increase (P<0.05) in the RCCAO+RBE group compared to the RCCAO group (Fig. 2).
Moreover, the number of the granular cells was significantly decreased (P<0.05) in the RCCAO group compared to the control one (61±2.89 vs. 124.16±1.69). However, it showed a significant increase (P<0.05) in the RCCAO+RBE group compared with the RCCAO group (116.83±2.75 vs. 61±2.89) (Table 1, Fig. 2).
In toluidine blue-stained sections, the cytoplasm of the granular cells of the control group was filled with dense dark blue Nissl’s granules. In the RCCAO group, the Nissl’s granules content appeared faint blue. In the RCCAO+RBE group, the Nissl’s granules content appeared dark blue (Fig. 3). Statistically, a significant decrease (P<0.001) in the color intensity was observed in the RCCAO group as compared to the control group (84.66±1.64 vs. 140.73±5.74). Even so, there was a significant increase (P<0.001) in the RCCAO+RBE group as compared to the RCCAO group (116.73±4.13 vs. 84.66±1.64) (Table 1, Fig. 3d).
Immunohistochemical results
In GFAP-stained sections, the RCCAO group showed a significant increase (p<0.001) in the number of astrocytes compared to the control group (30.66±4.5 vs. 3.16±1.61). In the RCCAO group treated with red beetroot extract, there was a significant decrease (p<0.001) in the number of astrocytes compared to the RCCAO group (12. 65±1.75 vs. 30.66±4.5) (Fig. 3a-c and Fig. 4a).
In HSP70 stained sections, the RCCAO group showed a significant increase (P<0.01) in the number of HSP positive cells compared to the control group (16.33±2.5 vs. 5.46±0.86). However, in RCCAO groups treated with beetroot extract, a more significant increase (P<0.001) in this percentage was observed compared to the RCCAO group (30.33±5.58 vs. 16.33±2.5) (Fig. 3d-f and Fig. 4b).
In Caspase-3-stained sections, the RCCAO group showed a significant increase (P<0.001) in the caspase positive cells compared to the control group (55.48±4.15 vs. 5.42±.17). In the RCCAO group treated with beetroot, a significant decrease (P<0.001) in this percentage was observed compared to the RCCAO group (34.75±1.17 vs. 55.48±4.15) (Fig. 3g-i, Fig. 4c).
In TNF-alpha stained sections, the RCCAO group showed a significant increase (P<0.001) in its area percentage compared to the control group (1.46±0.86 vs. 29.33±2.52). RCCAO groups treated with red beetroot extract showed a significant decrease (P<0.001) in area percentage of TNF-alpha compared to the RCCAO group (12.32±3.04.vs29.33±2.52) (Fig. 3j-l, Fig. 4d).
Biochemical results
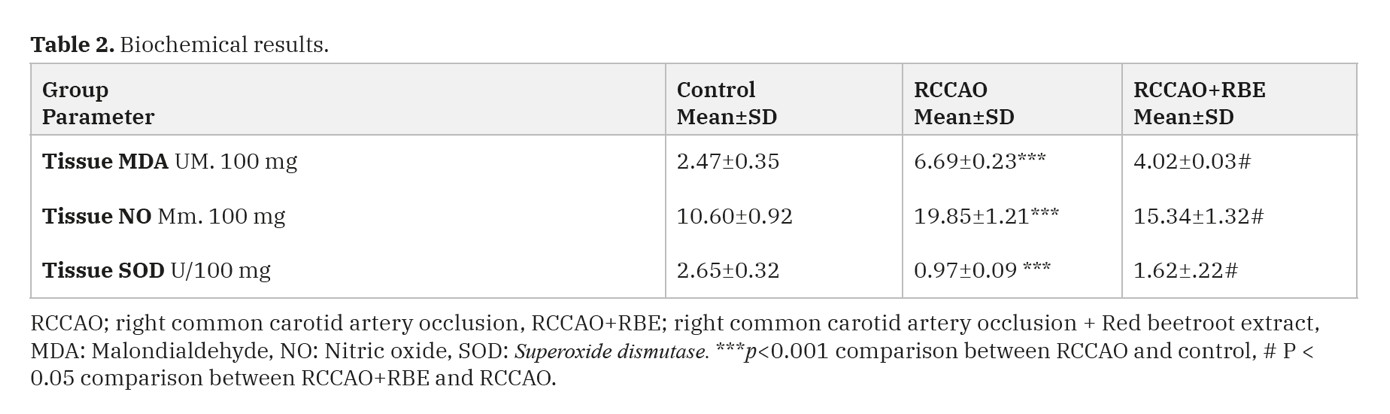
A significant increase in MDA level was observed in the RCCAO group (P<0.001) as compared with the control group. However, there was a significant decrease in its level in the RCCAO+RBE protected group (P<0.05) as compared with the RCCAO group (Table 2).
In this study we assessed the level of NO, which has an important role in the formation of oxidative damage and whose expression has also been detected in neural cells. There was a significant increase in NO level in the RCCAO group (p<0.001) compared to the control. It has been observed that red beetroot extract administration decrease (P<0.05) reperfusion injury and regresses the NO levels (Table 2).
Antioxidant marker tissue (SOD) activity is a major defense mechanism that protects the cells against oxidants. In this study, antioxidants decreased significantly in the RCCAO group (p<0.001) compared to the control group, while pretreatment with red beetroot extract decreases (P<0.05) reperfusion injury and regresses the NO levels (Table 2).
DISCUSSION
Ischemic stroke is one of the major leading causes of death and common causes of adult disability worldwide (Evenson et al., 1999). Considering the poor prognosis of ischemic stroke, protective therapies are being investigated to decrease neurological complications of its patients. Consequently, the present study was designed to investigate, for the first time to the best of our knowledge, the possible protective effect of red beetroot extract on the post-ischemic dentate gyrus neurodegeneration.
Examination of the dentate gyrus of the ischemia group showed small dark-stained nuclei in the granule cell layer and areas of cell loss. The same results were obtained by Irmak et al. (2003), who stated that cerebral ischemia up-regulated the expression of a transcription factor that has a pivotal role in mediating inflammatory response and reactive oxygen species (ROS) protein. This overproduction of ROS, inflammation, and oxidative stress leads to neuronal injury (Irmak et al., 2003) and increases the occurrence of apoptotic cell death in the brain (Chan, 2001; Choi-Kwon et al., 2004).
H&E-stained sections of the RCCAO group of our study reported numerous spindle-shaped cells in the subgranular zone (SGZ). These results were explained by Neuberger et al. (2017), who mentioned that after traumatic brain injury the hippocampal dentate gyrus acts as a focus of enhanced neurogenesis and excitability. This increment of neurogenesis has been proposed to help repair the injured granular cells.
Nissl granules in toluidine blue staining were used as a morphological indicator of neuronal survival. This study revealed a significant reduction in toluidine blue color intensity in the dentate gyrus. The same results were obtained by Guo et al. (2016).
We used immunohistochemistry to detect expressions of HSP-70, GFAP, Caspase-3, and TNF-alpha. Caspase-3 assay was used in order to detect the neuronal apoptosis, which was upregulated in the dentate gyrus of the RCCAO group. This follows Liu et al. (2013), who explained that ischemia/reperfusion injury enhanced caspase-3 activity or induced its expression, promoting neuron apoptosis.
The number of active GFAP-positive cells significantly increased in the dentate gyrus of the RCCAO group, and this agrees with Erfani et al. (2019), who stated that cerebral ischemia causes neuroinflammation intermediary activity of astrocytes in the hippocampus. These findings were confirmed by Turturici et al. (2011), who stated that astrocytic response or gliosis was a recognized response to an insult, which is typically characterized by an increase of polymerized GFAP. They added that increase GFAP expression was the major hallmark of the astroglial response to brain injury.
HSP-70 and TNF-α immunohistochemistry showed a statistically significant increased expression in the dentate gyrus of the RCCAO group. The release of TNF-α inflammatory molecules with other substances aggravates cell injury during ischemia (Gilmore, 2006). This agrees with previous studies in rat models. HSP-70 is a 70-kDa heat-shock protein stress-induced molecule that up-regulated in response to various types of CNS injuries, including stroke, trauma, or neurodegenerative disorders. Marked increase of HSP-70 in brain tissue of ischemic group induced by oxidative stress (Yasuda et al., 2011; Robinson et al., 2005).
Oxidative stress plays a major role in cerebral ischemic-reperfusion injury (Granger and Kvietys, 2015). In this study, oxidative stress in RCCAO rats, as indicated by significantly higher MDA and NO and significantly lower SOD in the RCCAO group than in the Control group, coincides with a previous study (Guven et al., 2015). MDA is the ultimate product and is one of the most sensitive indicators of lipid peroxidation (Gutteridge, 1995). Increased lipid peroxidation may result in the release of mitochondrial matrix enzymes and lysosomal proteolytic enzymes in the cytoplasm. So, intracellular proteolysis and cellular destruction increase (White et al., 1993). As a result, the immune system including SOD as an antioxidant enzyme involves a wide range of cellular activity to protect neuronal cells from ROS-induced cell death (Bosca and Hortelano, 1999). SOD detoxifies O2 to H2O2, which is then scavenged by peroxisomal catalase. In brief, H2O2 cannot be easily scavenged during ischemia due to the lower activity of SOD (Bosca and Hortelano, 1999). NO generated from neuronal NO synthase nitrosylates protein, which leads to cell dysfunction (Liu et al., 2008).
H&E-stained sections of the dentate gyrus of group III revealed that some of the granule cells regain their normal appearance (with rounded vesicular nuclei), while other cells had small dark nuclei. Vacuolation of the neuropil is still present in a small dose. These changes were also obtained by Bailey et al. (2009). Consuming beetroot juice as part of a high nitrate diet can improve the blood flow and oxygenation to the brain areas, so mediating inflammatory response and a less expressed and less overproduction of reactive oxygen species (ROS) protein, inflammation, and oxidative stress. This leads to less neuronal injury and decreases the occurrence of apoptotic cell death in the brain.
Less spindle-shaped cells were detected in SGZ. The same results obtained by Presley et al. (2003) added that red beetroot augmented neurogenesis in the ischemic brain and improved functional outcomes (Burdette et al., 2011). It attenuates neuroblast apoptosis and can enhance dentate gyrus neurogenesis by augmenting the survival and proliferation of the hippocampal neural progenitor cells (Toledano et al.,2012; Rockenstein et al., 2007). Thus, it plays a major role in promoting neurogenesis and gliogenesis (Nixdorf-Bergewiler et al.,1994).
In this study, a significant increase in toluidine blue color intensity in dentate gyrus was detected. The same results were obtained by Guo et al. (2016).
In immunohistochemistry of this group to detect Caspase-3 assay was downregulated in the dentate gyrus of the RCCAO+RBE group. This follows Liu et al. (2013), who explained that red beetroot contains nitrate, which makes vasodilatation, and so increases blood flow and inhibits ischemia/reperfusion injury by less expression of caspase-3.
TNF-α showed a significant decrease. Jin et al. (2013) stated that choline in beetroot helps to maintain the structure of cellular membranes, aids in the transmission of nerve impulses, assists in the absorption of fat, thus reducing inflammation.
Immunohistochemical results for GFAP-stained sections of group III revealed positive expression of GFAP in astrocytes. The same results were obtained by Yao et al. (2014), who stated that beetroot has a neurotrophic effect that might reduce the neurodegenerative alterations in Alzheimer’s disease (AD). On the other hand, Jin et al. (2013) stated that beetroot did not significantly increase the number of GFAP positive cells, but beetroot in a dose-dependently way enhances neurogenesis.
However, HSP-70 showed significantly more increase than the RCCAO group. The same results obtained by Robinson et al. (2005) concluded that HSP-70 may have anti-inflammatory, cytoprotective, and anti-apoptotic actions. Beetroot has an anti-inflammatory effect (Gilchrist et al., 2014).
Also, the same group showed a statistically significant decrease in MDA and NO and a significant increase in antioxidant SOD. The same findings were founded by Fulford et al. (2013). Gilchrist et al. (2014) suggest adaptive mechanisms to fight against neurodegeneration and added that beets contain an antioxidant known as alpha-lipoic acid, which may help prevention of oxidative stress-induced changes.
Conclusion
The results of this study confirm the neurodegenerative effects of cerebral ischemia-reperfusion on the rat dentate gyrus, add new information to the known neuroprotective effects of beetroot extract, and provide new insight into the possible use of beetroot extract to enhance these effects after ischemia.
Related articles
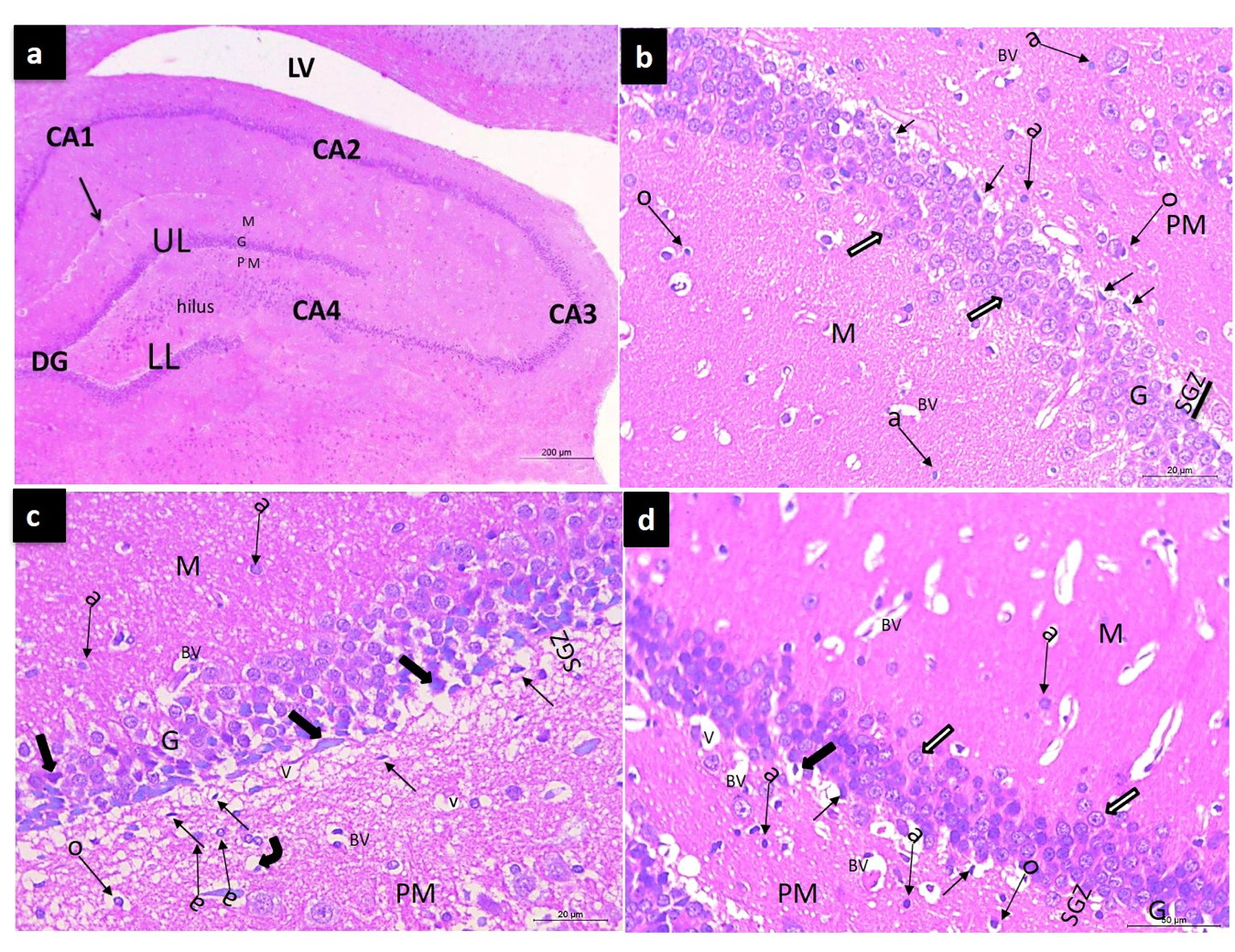 Fig. 1.- Representative photomicrographs (a) of coronal sections at the hippocampus and the dentate gyrus (DG), the dentate gyrus is formed of upper limb (UL), lower limb (LL), every limb formed of three: molecular (M), Granular (G) and polymorphic (PM) layers and stem in between, with a narrow hippocampal sulcus (arrow) in between. The hippocampus proper is composed of four areas: CA1, CA2, CA3, and CA4. The cavity of the lateral ventricle (LV). (b) The control group is showing the three layers of dentate gyrus: the molecular (M), Granular (G), and polymorphic (PM) layers. The Granular layer is formed of multiple regular rows of the Granular cells that appear tightly elliptical cells with basophilic cytoplasm (white arrows). Oligodendroglia; dark small nuclei and perinuclear halo (o), and astrocyte; oval vesicular nuclei (a) are scattered inside the neuropil. (c) The RCCAO group showing pyknotic granular cells (thick arrows), vacuolations (V), congested blood vessels (BV), degenerated oligodendroglia (bent arrow), and numerous astrocytes (a). the SGZ shows an increased number of spindle-shaped cells (thin arrows). (d) RCCAO group treated with beetroot extract showing many normal granular cells (white arrows) but some pyknotic (thick arrows) and are still present. Normal oligodendroglia (o), few astrocytes (a), few vacuolations (V), and a small number of spindle cells (thin arrows) of SGZ are observed within the neuropil. Scale bars: a = 200 µm); b, c, d = 20 µm, ×400).
Fig. 1.- Representative photomicrographs (a) of coronal sections at the hippocampus and the dentate gyrus (DG), the dentate gyrus is formed of upper limb (UL), lower limb (LL), every limb formed of three: molecular (M), Granular (G) and polymorphic (PM) layers and stem in between, with a narrow hippocampal sulcus (arrow) in between. The hippocampus proper is composed of four areas: CA1, CA2, CA3, and CA4. The cavity of the lateral ventricle (LV). (b) The control group is showing the three layers of dentate gyrus: the molecular (M), Granular (G), and polymorphic (PM) layers. The Granular layer is formed of multiple regular rows of the Granular cells that appear tightly elliptical cells with basophilic cytoplasm (white arrows). Oligodendroglia; dark small nuclei and perinuclear halo (o), and astrocyte; oval vesicular nuclei (a) are scattered inside the neuropil. (c) The RCCAO group showing pyknotic granular cells (thick arrows), vacuolations (V), congested blood vessels (BV), degenerated oligodendroglia (bent arrow), and numerous astrocytes (a). the SGZ shows an increased number of spindle-shaped cells (thin arrows). (d) RCCAO group treated with beetroot extract showing many normal granular cells (white arrows) but some pyknotic (thick arrows) and are still present. Normal oligodendroglia (o), few astrocytes (a), few vacuolations (V), and a small number of spindle cells (thin arrows) of SGZ are observed within the neuropil. Scale bars: a = 200 µm); b, c, d = 20 µm, ×400).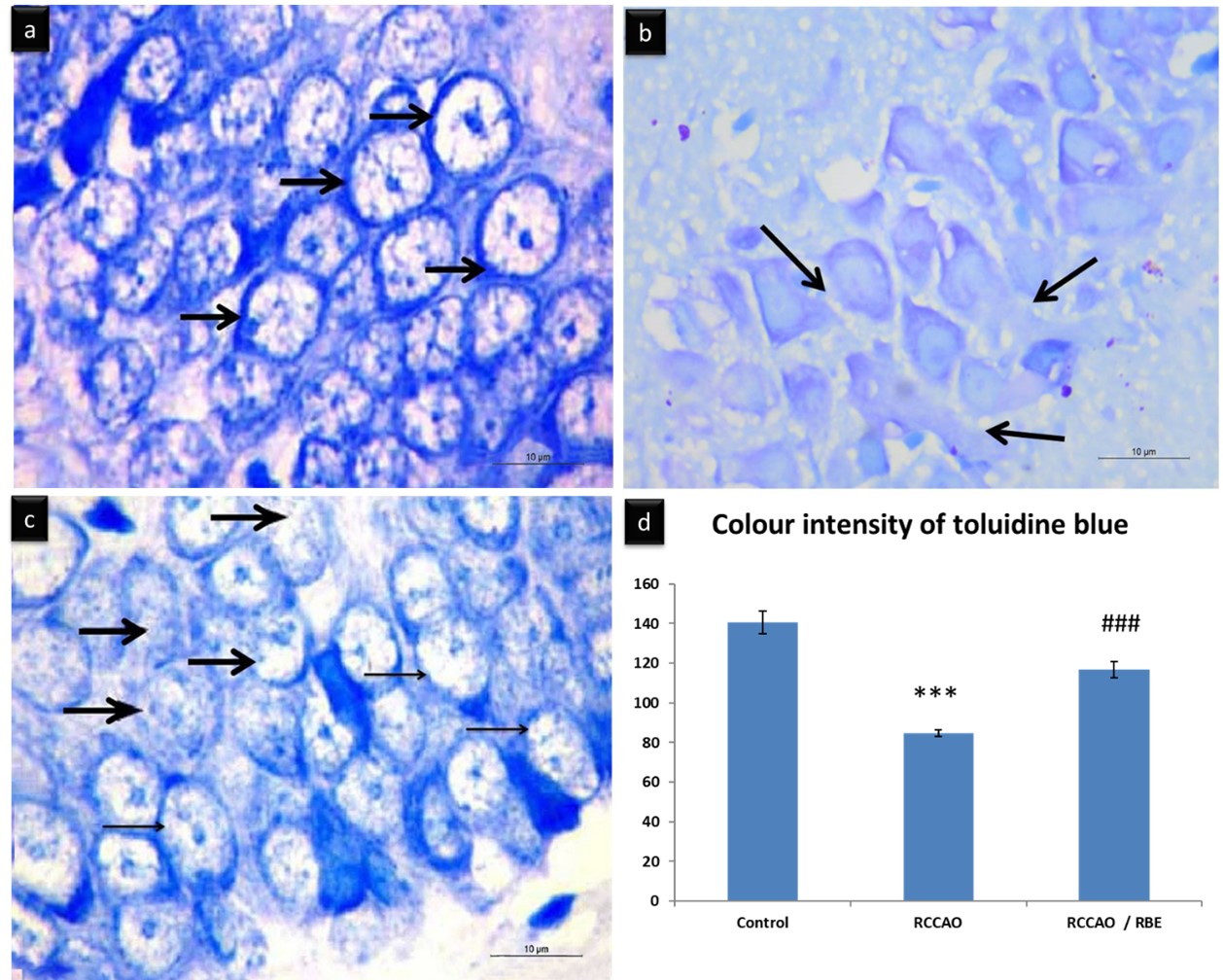 Fig. 2.- Representative micrographs of TB-stained sections in the dentate gyrus. (a) control group showing dense dark blue Nissl’s granules content inside the cytoplasm of the granular cells (thick arrows). (b) RCCAO group showing an apparent decrease of Nissl’s granules content inside the cytoplasm of the granular cells that appeared faint blue (arrows). (c) RCCAO+RBE treated with beetroot extract showing increased Nissl’s granules content in many of the granular cells which appeared dark blue (thin arrow) with only a few faint blue Nissl’s granules (thick arrow). Scale bars a-c = 10 µm, ×1000). (d) Mean color intensity (pixel) of Nissl’s granules. *** p < 0.001compared with control, ### P< 0.001compared with RCCAO.
Fig. 2.- Representative micrographs of TB-stained sections in the dentate gyrus. (a) control group showing dense dark blue Nissl’s granules content inside the cytoplasm of the granular cells (thick arrows). (b) RCCAO group showing an apparent decrease of Nissl’s granules content inside the cytoplasm of the granular cells that appeared faint blue (arrows). (c) RCCAO+RBE treated with beetroot extract showing increased Nissl’s granules content in many of the granular cells which appeared dark blue (thin arrow) with only a few faint blue Nissl’s granules (thick arrow). Scale bars a-c = 10 µm, ×1000). (d) Mean color intensity (pixel) of Nissl’s granules. *** p < 0.001compared with control, ### P< 0.001compared with RCCAO.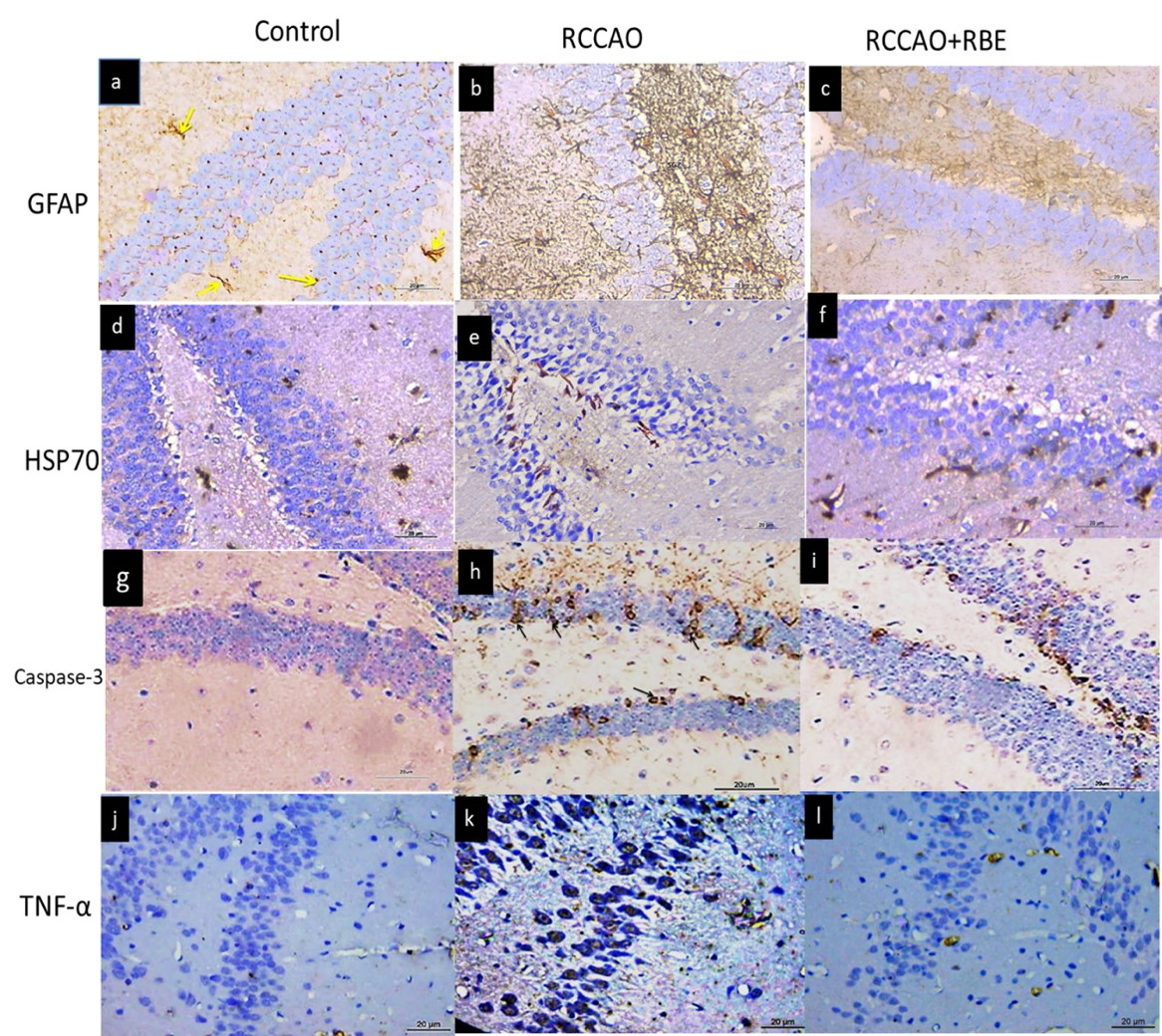 Fig. 3.- Representative micrographs of the different experimental groups showing significant upregulation of the GFAP (a-c), Caspase-3 (g-i), and TNF-α (j-l) immunoreaction in the RCCAO group and their downregulation in RCCAO+RBE group. HSP70 (d-f) shows significant slight upregulation in the RCCAO group and more upregulation in RCCAO+RBE group. Scale bars a-l = 20 µm, ×400).
Fig. 3.- Representative micrographs of the different experimental groups showing significant upregulation of the GFAP (a-c), Caspase-3 (g-i), and TNF-α (j-l) immunoreaction in the RCCAO group and their downregulation in RCCAO+RBE group. HSP70 (d-f) shows significant slight upregulation in the RCCAO group and more upregulation in RCCAO+RBE group. Scale bars a-l = 20 µm, ×400).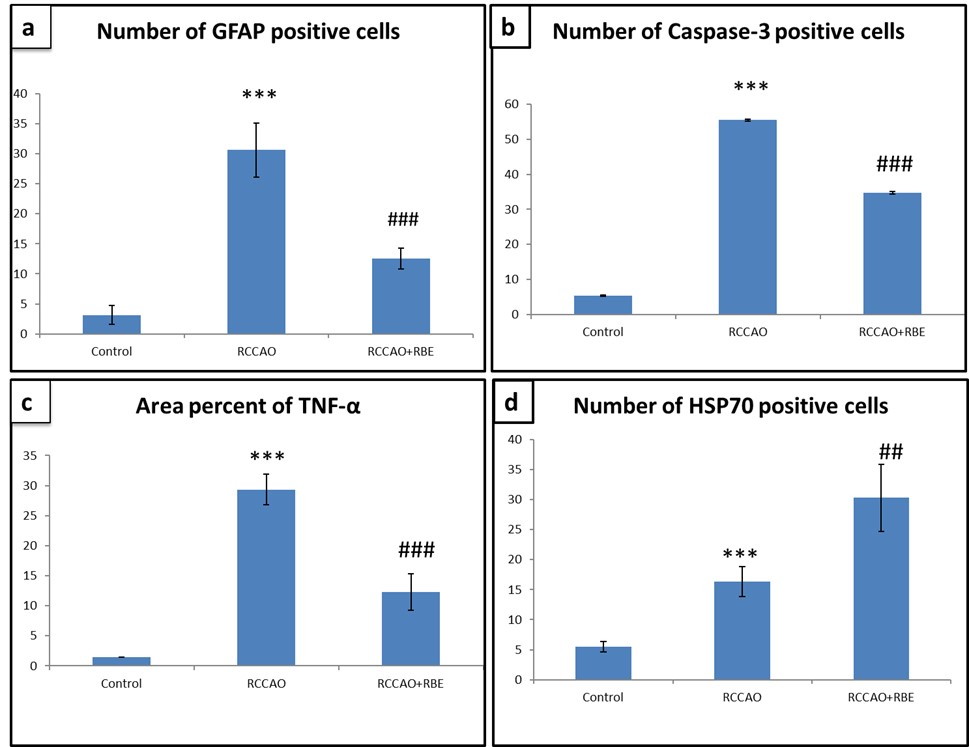 Fig. 4.- Histogram represent (a) Number of GFAP positive cells, (b) Number of Caspase-3 positive cells, (c) Area percent of TNF alpha immunoreaction and (d) Number of HSP 70 positive cells, *** p < 0.001 comparison between RCCAO and control groups; ## P < 0.01, ### P < 0.001 comparison between RCCAO+RBE and RCCAO groups.
Fig. 4.- Histogram represent (a) Number of GFAP positive cells, (b) Number of Caspase-3 positive cells, (c) Area percent of TNF alpha immunoreaction and (d) Number of HSP 70 positive cells, *** p < 0.001 comparison between RCCAO and control groups; ## P < 0.01, ### P < 0.001 comparison between RCCAO+RBE and RCCAO groups.ABD-ALLAH F, MOUSTAFA RR (2014) Burden of stroke in Egypt: current status and opportunities. Int J Stroke, 9(8): 1105-1108.
BAILEY SJ, WINYARD P, VANHATALO A, BLACKWELL JR, DIMENNA FJ, WILKERSON DP, TARR J, BENJAMIN N, JONES AM (2009) Dietary nitrate supplementation reduces the O2 cost of low-intensity exercise and enhances tolerance to high-intensity exercise in humans. J Appl Physiol, 107(4): 1144-1155.
BENAMER HT, GROSSET D (2009) Stroke in Arab countries: a systematic literature review. J Neurol Sci, 284(1-2): 18-23.
BOSCA L, HORTELANO S (1999) Mechanisms of nitric oxide-dependent apoptosis: involvement of mitochondrial mediators. Cell Signal, 11: 239-244.
CHAN PH (2001) Reactive oxygen radicals in signaling and damage in the ischemic brain. J Cereb Blood Flow Metab, 21: 2-14.
CHOI-KWON S, PARK KA, LEE HJ, PARK MS, LEE JH, JEON, SE, CHOE MA, PARK KC (2004) Temporal changes in cerebral antioxidant enzyme activities after ischemia and reperfusion in a rat focal brain ischemia model: effect of dietary fish oil. Dev Brain Res, 152(1): 11-18.
CLIFFORD T, HOWATSON G, WEST DJ, STEVENSON EJ (2015) The potential benefits of red beetroot supplementation in health and disease. Nutrients, 7(4): 2801-2822.
EL GAMAL AA, ALSAID MS, RAISH M, AL-SOHAIBANI M, AL-MASSARANI SM, AJAZ A, HEFNAWY M, AL-YAHYA M, OMER A, BASOUDAN OA, RAFATULLAH S (2014) Beetroot (Beta vulgaris L.) extract ameliorates gentamicin-induced nephrotoxicity associated oxidative stress, inflammation, and apoptosis in rodent model. Mediators Inflamm, 22(1): 938-952.
ERFANI S, MOGHIMI A, ABOUTALEB N, KHAKSARI M (2019) Protective effects of nucleobinding-2 after cerebral ischemia via modulating Bcl-2/Bax ratio and reducing glial fibrillary acid protein expression. Basic Clin Neurosci, 10(5): 451-460.
EVENSON KR, ROSAMOND WD, CAI J, TOOLE JF, HUTCHINSON RG, SHAHAR E, FOLSOM AR (1999) Physical activity and ischemic stroke risk. The atherosclerosis risk in communities study. Stroke, 30(7): 1333-1339.
FULFORD J, WINYARD PG, VANHATALO A, BAILEY SJ, BLACKWELL JR, JONES AM (2013) Influence of dietary nitrate supplementation on human skeletal muscle metabolism and force production during maximum voluntary contractions. Pflügers Arch, 465(4): 517-528.
GILCHRIST M, WINYARD PG, FULFORD J, ANNING C, SHORE AC, BENJAMIN N (2014) Dietary nitrate supplementation improves reaction time in type 2 diabetes: development and application of a novel nitrate-depleted beetroot juice placebo. Nitric Oxide, 40: 67-74.
GILMORE TD (2006) Introduction to NF-κB: players, pathways, perspectives. Oncogene, 25(51): 6680-6684.
GOLDSTEIN LB (2007): Acute ischemic stroke treatment in 2007. Circulation, 116: 1504-1514.
GRANGER DN, KVIETYS PR (2015) Reperfusion injury and reactive oxygen species: The evolution of a concept. Redox Biol, 6: 524-551.
GUO K, YIN G, ZI X, YAN W (2016) Activation of STAT3 is involved in neuronal apoptosis in focal cerebral ischemia/reperfusion rats via Bcl2/Fas pathway. Int J Clin Exp Pathol, 9(2): 2660-2669.
GUTTERIDGE JM (1995) Lipid peroxidation and antioxidants as biomarkers of tissue damage. Clin Chem, 41: 1819-1828.
GUVEN M, ARAS AB, AKMAN T (2015) Neuroprotective effect of p-coumaric acid in rat model of embolic cerebral ischemia. Iran J Basic Med Sci, 18(4): 356-363.
IRMAK MK, FADILLIOGLU E, SOGUT S, ERDOQAN H, GULEC M, OZER M (2003) Effects of caffeic acid phenethyl ester and alpha-tocopherol on reperfusion injury in rat brain. Cell Biochem Funct, 21: 283-289.
JIN R, LIU L, ZHANG S, NANDA A, LI G (2013) Role of inflammation and its mediators in acute ischemic stroke. J Cardiovasc Transl Res, 6(5): 834-851.
KIM AS, JOHNSTON SC (2011) Global variation in the relative burden of stroke and ischemic heart disease. Circulation, 124(3): 314-323.
LEE H, NAM YS, LEE KM (2015) Development-assistance strategies for stroke in low- and middle-income countries. J Korean Med Sci, Suppl 2: S139-142.
LIU G, WANG T, WANG T, SONG J, ZHOU Z (2013) Effects of apoptosis-related proteins caspase-3, Bax and Bcl-2 on cerebral ischemia rats. Biomed Rep, 1(6): 861-867.
LIU R, GAO M, YANG ZH, DU GH (2008) Pinocembrin protects rat brain against oxidation and apoptosis induced by ischemia–reperfusion both in vivo and in vitro. Brain Res, 1216: 104-115.
LOZANO R, NAGHAVI M, FOREMAN K, LIM S, SHIBUYA K, ABOYANS V, ABRAHAM J, ADAIR T, AGGARWAL R, AHN SY, ALVARADO M, ANDERSON HR, ANDERSON LM, ANDREWS KG, ATKINSON C, BADDOUR LM, BARKER-COLLO S, BARTELS DH, BELL ML, BENJAMIN EJ, BENNETT D (2013) Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet, 380(9859): 2095-2128.
MENTARI IA, NAUFALINA R, RAHMADI M, KHOTIB J (2018) Development ischemic stroke model by right unilateral common carotid artery occlusion (RUCCAO) method. Folia Medica Indonesiana, 54(3): 200.
MOZAFFARIAN D, BENJAMIN EJ, GO AS, ARNETT DK, BLAHA MJ, CUSHMAN M, DAS SR, DE FERRANTI S, DESPRÉS JP, FULLERTON HJ, HOWARD VJ, HUFFMAN MD, ISASI CR, JIMÉNEZ MC, JUDD SE, KISSELA BM, LICHTMAN JH, LISABETH LD, LIU S, MACKEY RH, MAGID DJ, MCGUIRE DK, MOHLER ER 3RD, MOY CS, MUNTNER P, MUSSOLINO ME, NASIR K, NEUMAR RW, NICHOL G, PALANIAPPAN L, PANDEY DK, REEVES MJ, RODRIGUEZ CJ, ROSAMOND W, SORLIE PD, STEIN J, TOWFIGHI A, TURAN TN, VIRANI SS, WOO D, YEH RW, TURNER MB, et al. (2016) American Heart Association Statistics Committee: A report from the American Heart Association. Circulation, 133(4): 447-454.
NEUBERGER EJ, SWIETEK B, CORRUBIA L, PRASANNA A, SANTHAKUMAR V (2017) Enhanced dentate neurogenesis after brain injury undermines long-term neurogenic potential and promotes seizure susceptibility. Stem Cell Reports, 9(3): 972-984.
NIXDORF-BERGEWILER BE, ALBRECHT D, HEINEMANN U (1994) Developmental changes in the number, size and orientation of GFAP-positive cells in CA1 region of rat hippocampus. Glia, 12: 180-195.
PANELKEVIN M, MULLANE R, KRAEMER BS (1985) Myeloperoxidase activity as a quantitative assessment of neutrophil infiltration into ischemie myocardium. J Pharmacol Meth, 14(3): 157-167.
PRESLEY TD, MORGAN AR, BECHTOLD E, CLODFELTER W, DOVE RW, JENNINGS JM, KRAFT RA, KING SB, LAURIENTI PJ, REJESKI WJ, BURDETTE JH, KIM-SHAPIRO DB, MILLER GD (2011) Acute effect of a high nitrate diet on brain perfusion in older adults. Nitric Oxide, 24(1): 34-42.
ROBINSON MB, TIDWELL JL, GOULD T, TAYLOR AR, NEWBERN JM, GRAVES J, MILLIGAN CE (2005) Extracellular heat shock protein 70: a critical component for motor neuron survival. J Neurosci, 25(42): 9735-9745.
TATEBAYASHI Y, LEE MH, LI L, IQBAL K, GRUNDKE-IQBAL I (2003) The dentate gyrus neurogenesis: a therapeutic target for Alzheimer’s disease. Acta Neuropathol, 105(3): 225-232.
TOLEDANO A, ALVAREZ MI, MONLEÓN E, TOLEDANO-DÍAZ A, BADIOLA JJ, MONZÓN M (2012) Changes induced by natural scrapie in the calretinin-immunopositive cells and fibres of the sheep cerebellar cortex. Cerebellum, 11(2): 593-604.
TURTURICI G, SCONZO G, GERACI F (2011) Hsp70 and its molecular role in nervous system diseases. Biochem Res Int, 2011: 618127.
VIRANI SS, ALONSO A, BENJAMIN EJ, BITTENCOURT MS, CALLAWAY CW, CARSON AP, CHAMBERLAIN AM, CHANG AR, CHENG S, DELLING FN, DJOUSSE L, ELKIND MSV, FERGUSON JF, FORNAGE M, KHAN SS, KISSELA BM, KNUTSON KL, KWAN TW, LACKLAND DT, LEWIS TT, LICHTMAN JH, LONGENECKER CT, LOOP MS, LUTSEY PL, MARTIN SS, MATSUSHITA K, MORAN AE, MUSSOLINO ME, PERAK AM, ROSAMOND WD, ROTH GA, SAMPSON UKA, SATOU GM, SCHROEDER EB, SHAH SH, SHAY CM, SPARTANO NL, STOKES A, TIRSCHWELL DL, VANWAGNER LB, TSAO CW, et al. (2020) American Heart Association Council on Epidemiology and Prevention Statistics Committee and Stroke Statistics Subcommittee. Heart Disease and Stroke Statistics-2020 update: A report from the American Heart Association. Circulation, 141(9): e139-e596.
WHITE BC, GROSSMAN LI, KRAUSE GS (1993) Brain injury by global ischemia and reperfusion: a theoretical perspective on membrane damage and repair. Neurology, 43: 1656-1665.
YAO Y, CHEN ZL, NORRIS EH, STRICKLAND S (2014) Astrocytic laminin regulates pericyte differentiation and maintains blood brain barrier integrity. Nat Commun, 5: 3413.
YASUDA Y, SHIMODA T, UNO K, TATEISHI N, FURUYA S, TSUCHIHASHI Y, FUJITA S (2011) Temporal and sequential changes of glial cells and cytokine expression during neuronal degeneration after transient global ischemia in rats. J Neuroinflamm, 8(1): 70.