The major drawback of the efforts currently being made to domesticate grasscutters (GRCs) is the paucity of data on their biology particularly in connection with sensory related reactions. We investigated, both grossly and histologically, the qualitative and quantitative features of the GRC nasal and brain olfactory components with a view to understanding their morphofunctional adaptations. At the caudal roof of the nasal cavity, the olfactory mucosa (OM), whose basic histology was similar to that of other mammals, covered a set of five cylindrical-shaped ethmoturbinates. Olfactory bulb (OB) and olfactory brain components (OBC) (i.e., OB, olfactory tract and stria) constituted 0.55 and 1.26% of the entire brain volume respectively. Relative to the cerebral hemispheres, respective proportions for lengths and breadths were 72.23% for OBC and 34.38% for OB. Regarding the OM, epithelial height and axon bundle diameters were 76.3 ± 5.4 µm and 70.6 ± 8.6 µm respectively while volume fractions were 31.2 ± 4.4% for the bundles and 49.7 ± 5.1% for Bowman’s glands. These findings reveal that the anatomical design of the GRC OM and OBC follows the general patterns observed in other mammals and are refined to levels that appear to correspond to demands in olfactory function vis-à-vis behavior and ecology.
Gross and histological study of nasal and brain olfactory structures in the grasscutter (Thryonomys swinderianus, Temminck)
Boniface Kavoi1,2, Rodi Ojoo1,2, Kwadwo Boateng2,3, Stephen Kiama1
1 Department of Veterinary Anatomy and Physiology, Faculty of Veterinary Medicine, University of Nairobi, Riverside Drive, Nairobi, Kenya
2 School of Veterinary Medicine, College of Applied and Basic Sciences, University of Ghana, Legon, Accra, Ghana
3 Animal Research Institute, Council for Scientific and Industrial Research, Achimota, Accra, Ghana
SUMMARY
Sign up or Login
INTRODUCTION
The grasscutter (GRC) (Thryonomys swinderianus, Temminck 1827), also known as the great cane rat or marsh cane-rat, is a rodent but not a rat proper (Opara, 2010). Rodents constitute the largest group among the mammalian orders, with about 2,277 species (Aydin et al., 2008). The GRC is indigenous to Africa and belongs to a small group of the so called African hystricognath rodents (Woods and Kilpatrick, 2005). Hystricomorpha, which are distinguished from other rodents by their skull structure, comprise of the families Thryonomydae (family of the GRCs), Hystricidae (porcupines), Bathyergidae (sand-diggers) and Petromuridae (African rock-rats) (Opara, 2010).
GRCs are prevalent in the West African sub-region from Senegal to parts of the Cape Province including Ghana, Nigeria, Togo, Benin, and Côte d’Ivoire (Rosevear, 1969). In these places, GRCs are hunted aggressively for their meat, which the locals consider a delicacy (Matthews, 2008). Consequently, in the aforementioned countries, relentless efforts are being made to domesticate GRCs by raising them in cages for home consumption and for sale (Fa et al., 2002). Indeed, GRCs in these regions are considered “micro livestock” (Karikari and Nyameasem, 2009) or more precisely “livestock of tomorrow” (Opara, 2010). Moreover, rearing and breeding of captive GRCs for use as research models are becoming habitual practice (Jori et al., 2001; Ibe et al., 2017).
In the wild, GRCs inhabit reed-beds and riverbanks where they live in small groups led by a single male (Woods and Kilpatrick, 2005). These animals are nocturnal monogastric herbivores that live above the ground and feed on roots, shoots, and stems of various grasses (Williams et al., 2011). GRCs are considered wasteful feeders in that they cut grass with a characteristic tooth-chattering sound to obtain the more nutritious succulent inner nodes, leaving behind scattered pieces on the ground (Asibey, 1974). Humans have expanded into the GRCs’ native habitats and this has forced GRCs to look for food and space in plantations (Opara, 2010). GRCs attain sexual maturity at the age of 7 months, when their body mass is 1.6-2.1 kg (Adu and Yeboah, 2003). In terms of appearance, GRCs have a thickset body, rounded ears, short nose, coarse bristle hair, spiny fur on the back (Fig. 1) and forefeet that are smaller than the hind. The body coat comprises a mixture of brown reddish and gray fur that varies depending on habitat (Jori and Chardonnet, 2001).
Data on the anatomy of the GRC is essential in understanding the basis for some of the behaviors it exhibits in its natural habitat, and which must be considered for its effective performance when reared and bred in cages. The anatomy of the GRC brain has been studied previously (Byanet et al., 2009; Ajayi et al., 2011; Byanet and Dzenda, 2014; Ibe et al., 2017), with little attention to the olfactory system. A study detailing the structure of reflex centers in the GRC brain revealed a relatively bigger caudal than rostral colliculi (Ibe et al., 2017), implying a better auditory than visual acuity. Besides, Opara (2010) opined that the poor visual acuity in the GRC makes communication to occur more effectively via auditory and olfactory senses. In this study, therefore, the gross, histologic and morphometric features of the GRC olfactory mucosa (OM) and olfactory brain components (OBC) are analyzed in an attempt to understand the neural substrates responsible for the reportedly better olfactory cue. Notably, results of this work add on to the growing literature on GRC anatomy. Furthermore, quantitative data recorded here may serve as an important taxonomic exponent of animals in the order Rodentia.
MATERIALS AND METHODS
Experimental animals
A total of ten healthy male captive-bred GRCs aged 13-15 months (2.8-3.2 kg bwt) were used. These were chosen from a colony raised in a conventional animal housing facility. The GRCs were transported from the housing facility in well ventilated wooden cages to the School of Veterinary Medicine, University of Ghana, anatomy laboratory, where they were immediately euthanized using pentobarbital sodium (140 mg/kg, intravenously). Perfusion fixation of the olfactory mucosae and brains was carried out through the left ventricle with saline followed by 10% formaldehyde. All procedures performed on the GRC were approved by the University’s Animal Care and Use Committee and were in strict conformity to the guidelines provided in the Animals (Scientific Procedures) Act 1986.
Harvesting of the brains
Soon after perfusion fixation, the brains (n= 5 animals) were harvested as detailed in Ibe et al. (2017). In brief, the head was cut off from the rest of the body at the atlanto-axial joint using a sharp knife. This was followed by skinning and stripping off the cranial and facial muscles, and then breaking the skull to extract the brain in caudorostral and dorsoventral directions using scalpel blades, thumb forceps and a pair of scissors. The brain was then allowed to fix further by immersing it overnight in 10% formaldehyde.
Harvesting and processing of the olfactory mucosa for histology
The ethmoidal conchae, onto which the olfactory mucosa (OM) lies, were harvested from the nasal cavity (n = 5 animals) following mid-sagittal sectioning of the skull and subsequent exposure of the conchae by dissecting out the nasal septum (Kavoi et al., 2010). The conchae were then transected perpendicular to their long axes into posterior, middle and anterior portions, from which tissue sub-segments for histology were selected by systematic random sampling.
The selected conchal pieces were decalcified in 5% EDTA (Alers et al., 1999), washed in distilled water and dehydrated in graded ethanol series (50, 70, 80, 90% and twice in 100%). This was followed by paraffin embedding and sectioning in the transverse plane at 5 μm using a rotary microtome (Leitz Wetzlar, Germany). Staining was then done in hematoxylin and eosin and Masson’s trichrome using routine procedures.
Morphometric analysis
Volume and linear measurements were performed on the brain as illustrated in Kavoi and Jameela (2011). The volumes for the whole brain (WB), cerebral hemisphere (CH), olfactory bulb (OB) and olfactory bulb, tract and stria combined (OBC) were determined using the water displacement method (Scherle, 1970).
Linear measurements (greatest lengths and breadths) were carried out on the OB, OBC and CH as illustrated in Fig. 2. Vernier calipers, thread and meter rule were used to take the measurements and three trained technicians were involved in the exercise (intra and inter-observer errors = 2-3%).
Quantitative parameters of the OM were analyzed from 30-35 test fields generated from randomly selected histomicrographs (Kavoi et al., 2010; Kavoi et al., 2012). The parameters estimated included: 1) thickness of the olfactory epithelium, 2) diameter of axon bundles and 3) volume fractions of axon bundles and Bowman’s glands. The protocols followed in carrying out these measurements are well outlined in Kavoi et al. (2012).
Data on morphometry were expressed as mean ± SD (standard deviation) and presented in tables. Comparisons of measurement values between OBC and the cerebrum or whole brain were expressed as ratios (%).
RESULTS
Morphology
Grossly, the GRC OM was identified by its characteristic yellowish-brown color at the caudal roof of the nasal cavity, where it was covered by a set of five cylindrical ethmoturbinates (Fig. 3).
It is noted in Fig. 4 that the histological appearance of the GRC OM was basically similar to that of other mammalian species. The epithelium (Fig. 4A) was organized into a free zone, non-nuclear zone and nuclear zone, with the latter zone having an upper region of elongated supporting cell nuclei, middle portion of rounded olfactory cell nuclei and a lower row of basal cell nuclei. Embedded in the lamina propria (Fig. 4B) were Bowman’s glands and olfactory nerve axon bundles. Here, the glands were of the mucus tubuloalveolar type, and were mainly confined in the upper two thirds of the propria. The lower third of the propria was predominated by axon bundles.
Morphometry
Volume measurements gave a value of 0.06 ± 0.008 cm3 for OBs, 0.14 ± 0.05 cm3 for OBC and 11.14 ± 0.72 cm3 for CH (Table 1). This translates to volume ratios of 0.55 and 1.26% for OB: CH and OBC: CH respectively.
Linear dimensions for olfactory brain structures are shown in Table 2. In terms of length, the OBC was 23.51 ± 1.23 mm long while CH measured 32.55 ± 1.86 mm, thus giving an OBC: CH ration of 72.23%. For the breadths, values for OB and CH were 5.56 ± 0.81 and 16.17 ± 1.54, respectively, and therefore the OB: CH ratio was 34.38%.
In Table 3, respective values for olfactory epithelial height and axon bundle diameter were 76.3 ± 5.4 and 70.6 ± 8.6 µm, whereas volume fractions of Bowman’s glands and axon bundles were 49.7 ± 5.1 and 31.2 ± 4.4 % respectively.
GRC data on lengths, breadths and volumes of olfactory structures with respect to the whole brain (in ratios %) are compared with those obtained earlier in other species in Table 4.
DISCUSSION
GRCs are African hystricognath rodents prevalent throughout sub-Saharan Africa (Ibitoye et al., 2019). In West African countries, GRC flesh is very popular for domestic consumers, and as such GRCs are currently being captured from the wild for breeding as minilivestock and research models (Ibe et al., 2017). Indeed, GRC domestication has significantly impacted on the livelihood of rural communities in West Africa (Adu, 2002; Adedapo and Adekunle, 2013). This study explores the anatomy of structures of the GRC olfactory system in an effort to understand the adaptive physiology of the animal. The olfactory system is the epitome of communication, playing a critical role in the regulation of social, sexual, maternal and feeding behaviors in most mammals (Mota-Rojas et al., 2018). Olfaction first occurs in the cilia of the olfactory neurons in the mammalian nasal cavity and the generated signals are then transmitted to the olfactory cortex via synaptic connections with downstream neurons in the olfactory bulb (Field et al., 2003). To the best of our knowledge, this is the first study to describe and quantify anatomical features of the olfactory system in the GRC.
At the caudal roof of GRC nasal cavity, the OM covers a set of five cylindrical-shaped ethmoturbinates. Although the number of the ethmoturbinates did not differ much from that of other mammals, the complexity, shape and orientation varied from that of some mammals. In dogs (Kavoi et al., 2010), the ethmoturbinates form prominent lamellar folds that serve to elaborate the surface area covered by the OM, whereas in the naked mole rat (Onyono et al., 2017) the turbinates are less complex, and present tongue- and flap-like shapes. In the root rat (Onyono et al., 2017), the turbinates are highly branched and quite extensive, occupying dorsomedial, medial and lateral surfaces of the nasal cavity. Although assessment of olfactory acuity would best be based on finer structural details such as packing densities of olfactory cells and cilia numbers per olfactory neuron (Kavoi et al., 2010, 2012), the size and degree of folding of the olfactory area are considered important indicators of olfactory capability (Pihlström et al., 2005).
Histologically, cell arrangement in the GRC OM epithelium takes the usual mammalian pattern in which basal cell are placed lowermost, followed by olfactory cells and then supporting cells. The thickness of the GRC OM epithelium was estimated at 76.3 µm, a value that was higher than that recorded in rats (genus rattus) (Sakashita et al., 1995), rufous sengi (Kavoi, 2018), sheep and dogs (Kavoi et al., 2010), but lower when compared to rabbits (Kavoi et al., 2012), root rats and mole rats (Onyono et al., 2017). Within the lamina propria of the OM, volume fraction of axon bundles was 31.2%, a value that was less than that reported in the rabbit (Kavoi et al., 2012). Olfactory sensitivity has been shown to increase with increasing OM epithelial thickness (Raimund et al., 1991) and axon bundles size (Van Drongelen et al., 1978). We therefore presume that the above differences in morphometric values signify variations in odor detection abilities vis-à-vis olfaction needs and ecological adaptations among species.
Parameters of specific brain parts show differences that reflect functional requirements between and within species (Kaas and Collins, 2001). On the GRC brain, linear dimensions and volumes of OBCs were determined and correlated with those of the cerebrum and the entire brain. The ratios of OBC to that of the cerebrum in regard to length and breadth was 72.23 and 34.38% respectively. These values exceed those previously reported in the rufous sengi (Kavoi and Kisipan, 2019), human and goat (Kavoi and Jameela, 2011) but are less than in the dog (Kavoi and Jameela, 2011). Regarding volume, the GRC OB: brain and OBC: brain volume ratios were greater than in all the above-mentioned species except for the dog (Kavoi and Jameela, 2011) in which the ratio was greater. Kaas (2000) attributes changes in parameters of certain brain parts to the number of neurons, the size of such neurons and/or their connectivity and, indeed, Haehner et al. (2008) showed OB volume to increase with olfaction ability.
In conclusion, results of this work show the GRC to have an olfactory system whose structural design is much more sophisticated/better specialized than that of many other mammals. The OM and OBS in this species are refined, both quantitatively and qualitatively, to levels that appear to correspond to olfaction needs pertaining to their behavior and ecology. As GRCs continue to receive more and more attention during domestication and use as research models, more anatomical work will be required to contribute to the understanding of the morpho-functional dynamics of its various organ systems. It is hoped that results of this investigation constitute a significant addition to the available database on the GRC anatomy. Moreover, data obtained here may find application in the broader areas of comparative anatomy and wildlife behavior. Future works should analyze the GRC olfactory system for ultrastructural characteristics.
ACKNOWLEDGEMENTS
We thank the Department of Animal Science, University of Ghana, for providing the GRCs for this work. Laboratory space, equipment and chemicals were made available by the School of Veterinary Medicine, University of Ghana, and also the Departments of Human Anatomy and Veterinary Anatomy and Physiology, University of Nairobi. Messrs. Derrick Asare, Kingsley Amoako and Ephraim Yawson helped in tissue harvesting.
Related articles
 Fig. 1.- Photographs showing characteristic body features of grasscutters (Thryonomys swinderianus). Notice the spiny fur on the back of its thickset body, large rounded ears, short nose and prominent vibrissae.
Fig. 1.- Photographs showing characteristic body features of grasscutters (Thryonomys swinderianus). Notice the spiny fur on the back of its thickset body, large rounded ears, short nose and prominent vibrissae.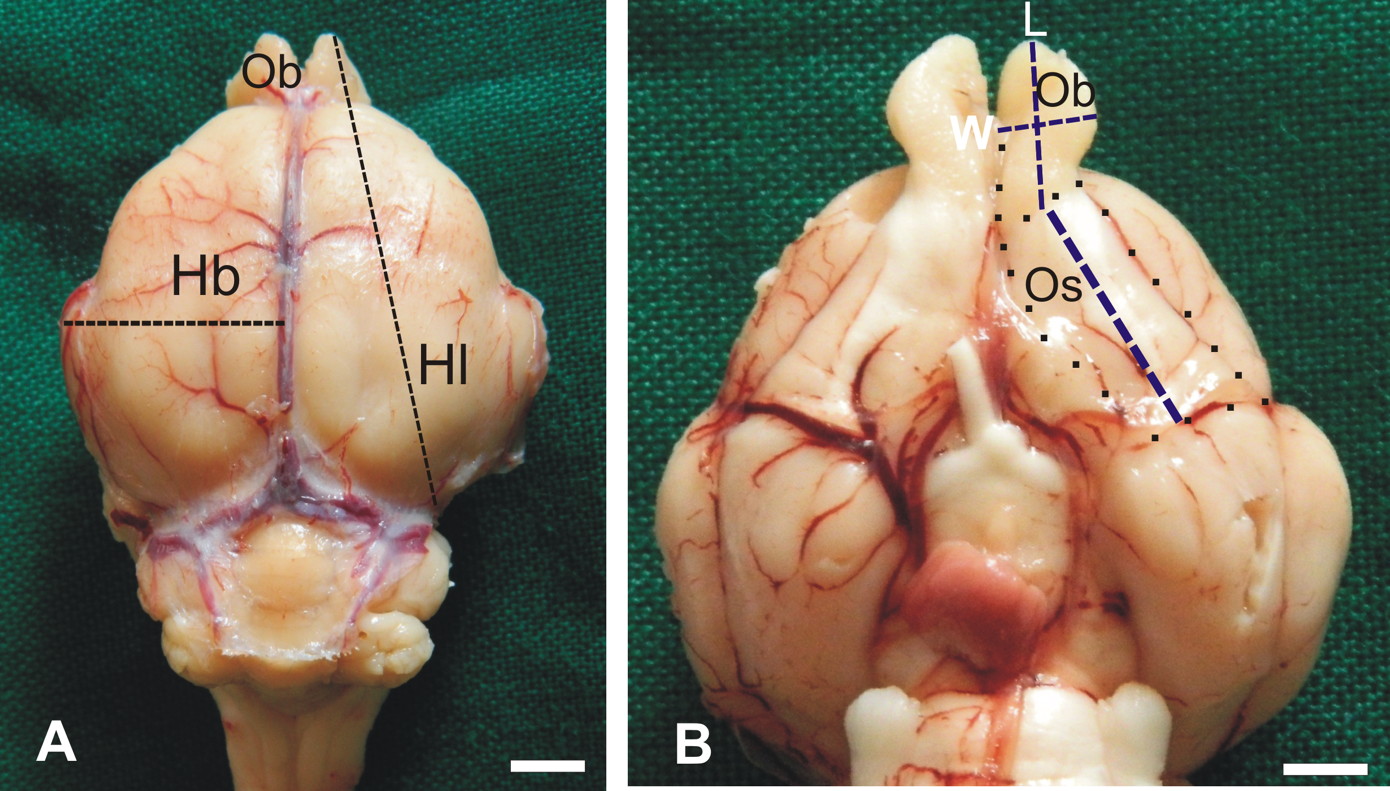 Fig. 2.- Macrographs showing how linear dimensions were estimated on the GRC brain. A: greatest length (Hl) and breadth (Hb) of the cerebral hemisphere were measured from the dorsal aspect of the brain. B: ventrally on the brain, the olfactory bulb (Ob) and olfactory tract and stria (Os) were dissected out (dotted lines) to allow measurement of their greatest lengths (L) and breadths (W). Scale bar = 5 mm in A and B.
Fig. 2.- Macrographs showing how linear dimensions were estimated on the GRC brain. A: greatest length (Hl) and breadth (Hb) of the cerebral hemisphere were measured from the dorsal aspect of the brain. B: ventrally on the brain, the olfactory bulb (Ob) and olfactory tract and stria (Os) were dissected out (dotted lines) to allow measurement of their greatest lengths (L) and breadths (W). Scale bar = 5 mm in A and B. 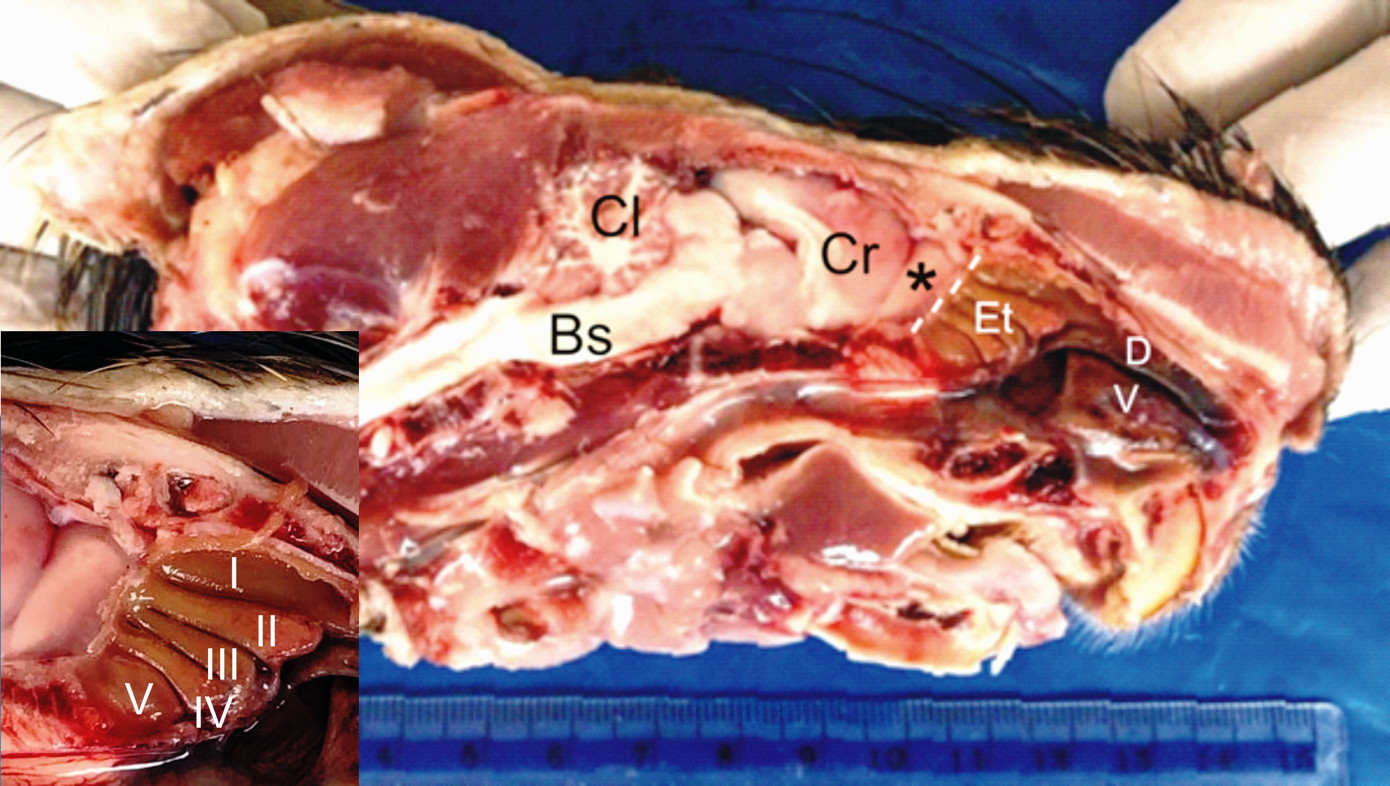 Fig. 3.- Macrograph of the left side of split head of GRC showing the location of the ethmoturbinates (Et) onto which the olfactory mucosa lies. The turbinates (inset) occur in a set of five and are cylindrical in shape. Cr- stand for cerebrum, Cl- cerebellum, Bs- brain stem, D- dorsal nasal concha, V- ventral nasal concha and (*)- olfactory bulb.
Fig. 3.- Macrograph of the left side of split head of GRC showing the location of the ethmoturbinates (Et) onto which the olfactory mucosa lies. The turbinates (inset) occur in a set of five and are cylindrical in shape. Cr- stand for cerebrum, Cl- cerebellum, Bs- brain stem, D- dorsal nasal concha, V- ventral nasal concha and (*)- olfactory bulb.  Fig. 4.- Light micrographs of the GRC olfactory mucosa. A: the epithelium is constituted by a nuclear zone (NZ), a non-nuclear zone (Nnz) and a mucociliary complex (arrow). The NZ has three regions: an upper region of elongated supporting cell nuclei (Scn), a middle region of olfactory cell nuclei (Ocn) and lower row of basal cell nuclei (Bcn) located just above the basement membrane (asterisks). B: the lamina propria containing tubuloacinar Bowman’s glands (Bg), olfactory nerve axon bundles (Nb) and blood vessels (Bv), with the bundles taking a deeper position than the glands. A- H&E stain, B- Masson’s trichrome stain. Scale bar = 20 µm in A, B.
Fig. 4.- Light micrographs of the GRC olfactory mucosa. A: the epithelium is constituted by a nuclear zone (NZ), a non-nuclear zone (Nnz) and a mucociliary complex (arrow). The NZ has three regions: an upper region of elongated supporting cell nuclei (Scn), a middle region of olfactory cell nuclei (Ocn) and lower row of basal cell nuclei (Bcn) located just above the basement membrane (asterisks). B: the lamina propria containing tubuloacinar Bowman’s glands (Bg), olfactory nerve axon bundles (Nb) and blood vessels (Bv), with the bundles taking a deeper position than the glands. A- H&E stain, B- Masson’s trichrome stain. Scale bar = 20 µm in A, B.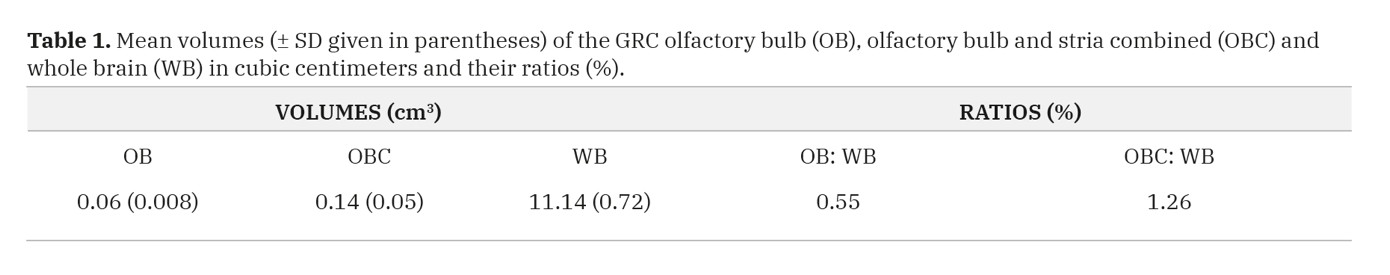 Table 1. Mean volumes (± SD given in parentheses) of the GRC olfactory bulb (OB), olfactory bulb and stria combined (OBC) and whole brain (WB) in cubic centimeters and their ratios (%).
Table 1. Mean volumes (± SD given in parentheses) of the GRC olfactory bulb (OB), olfactory bulb and stria combined (OBC) and whole brain (WB) in cubic centimeters and their ratios (%).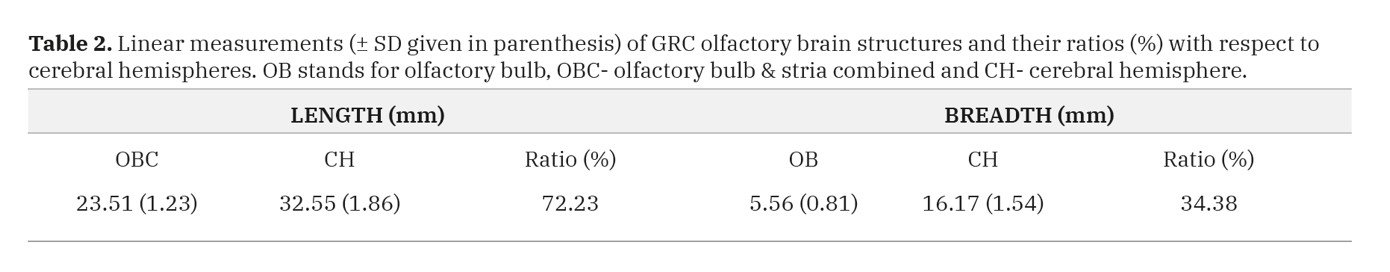 Table 2. Linear measurements (± SD given in parenthesis) of GRC olfactory brain structures and their ratios (%) with respect to cerebral hemispheres. OB stands for olfactory bulb, OBC- olfactory bulb & stria combined and CH- cerebral hemisphere.
Table 2. Linear measurements (± SD given in parenthesis) of GRC olfactory brain structures and their ratios (%) with respect to cerebral hemispheres. OB stands for olfactory bulb, OBC- olfactory bulb & stria combined and CH- cerebral hemisphere. Table 3. Mean values (± SD given in parenthesis) for olfactory epithelial thickness and axon bundle diameter (in µm) and volume fractions (%) of Bowman’s glands and axon bundles in the GRC.
Table 3. Mean values (± SD given in parenthesis) for olfactory epithelial thickness and axon bundle diameter (in µm) and volume fractions (%) of Bowman’s glands and axon bundles in the GRC. Table 4. Comparison of GRC brain morphometric parameters (ratios %) with those recorded earlier in the rufous sengi (Kavoi and Kisipan, 2019) and in the dog, goat and humans (Kavoi and Jameela, 2011). OBS, CH and WB denote olfactory brain components, cerebral hemisphere and whole brain, respectively.
Table 4. Comparison of GRC brain morphometric parameters (ratios %) with those recorded earlier in the rufous sengi (Kavoi and Kisipan, 2019) and in the dog, goat and humans (Kavoi and Jameela, 2011). OBS, CH and WB denote olfactory brain components, cerebral hemisphere and whole brain, respectively.ADEDAPO A, ADEKUNLE A (2013) Economic aspects of grasscutter farming in Southwest Nigeria: Implications for sustainable adoption and conservation. IJSER, 4: 17-23.
ADU EK (2002) Research on grasscutter production in Ghana. In: Atta-Agyepong K, Weidinger R (eds). Proceedings of a Workshop on Promoting Grasscutter Production for Poverty Reduction in Ghana (16-18 October, 2002), Sunyani, Ghana.
ADU EK, YEBOAH S (2003) On the use of perineal stain as an index of sexual maturity and breeding condition in the male greater cane rat (Thryonomys swinderianus, Temminck). Trop Anim Health Prod, 35: 433-439.
AJAYI IE, OJO SA, ONYEANUSI BI, GEORGE BDJ, AYO JO, SALAMI SO, IBE CS (2011) Gross observations and morphometry of the medulla oblongata of the African grasscutter (Thryonomys swinderianus). Vet Rec, 4: 5-8.
ALERS JC, KRIJTENBERG PJ, VISSERS KJ, VAN DEKKEN H (1999) Effect of bone decalcification procedures on DNA in situ hybridization and comparative genomic hybridization: EDTA is highly preferable to a routinely used acid decalcifier. J Histochem Cytochem, 47: 703-710.
ASIBEY EOA (1974) The grasscutter (Thryonomys swinderianus Temminck) in Ghana. Symp Zool Soc Lond, 34: 161-170.
AYDIN A, YILMAZ S, OZKAN ZE, ILGUN R (2008) Morphological investigations on the circulus arteriosus cerebri in mole-rats (Spalax Leucodon). Anat Histol Embryol, 37: 219-222.
BYANET O, DZENDA T (2014) Quantitative biometry of body and brain in the Grasscutter (Thryonomys swinderianus) and African giant rat (Cricetomys gambianus): encephalization quotient implication. Res Neurosci, 3: 1-6.
BYANET O, ONYEANUSI BI, IBRAHIM NDG (2009) Sexual dimorphism with respect to the macro-morphometric investigations of the forebrain and cerebellum of the grasscutter (Thryonomys swinderianus). Int J Morphol, 27: 361-365.
FA JE, JUSTE J, BURN RW, BROAD G (2002) Bushmeat consumption of two ethnic groups in Bioko Island, West Africa. Hum Ecol, 30: 397-416.
FIELD PM, LI Y, RAISMAN G (2003) Ensheathment of the olfactory nerves in the adult rat. J Neurocytol, 32: 317-324.
HAEHNER A., RODEWALD A, GERBER JC, HUMMEL T (2008) Correlation of olfactory function with changes in the volume of the human olfactory bulb. Arch Otolaryngol Head Neck Surg, 134: 621-624.
IBE CS, IKPEGBU E, NZALAK O (2017) Relationship between age and brain stem allometry in the African grasscutter (Thryonomys swinderianus Temminck, 1827). J S Afr Vet Assoc, Vol 88.
IBITOYE O, KOLEJO O, AKINYEMI G (2019) Burgeoning and domestication of grasscutter (Thryonomys swinderianus) in a post-Ebola era: A reassessment of its prospects and challenges in Nigeria. World Sci News, 130: 216-237.
JORI F, CHARDONNET P (2001) Cane rat farming in Gabon. Status and Perspective. Proceedings of the 5th International Wildlife Ranching Symposium. Pretoria, South Africa, pp 33-51.
JORI F, COOPER JE, CASAL J (2001) Postmortem findings in captive cane rats (Thryonomys swinderianus) in Gabon. Vet. Rec, 148: 624-628.
KAAS JH (2000) Why is brain size so important: design problems and solutions as neocortex gets bigger or smaller. Brain Mind, 1: 7-23.
KAAS JH, COLLINS CE (2001) Evolving ideas of brain evolution. Nature, 411: 141-142.
KARIKARI PK, NYAMEASEM JK (2009) Productive performance and carcass characteristics of captive grasscutters (Thryonomys swinderianus) fed concentrate diets containing varying levels of guinea grass. World Appl Sci J, 6: 557-563.
KAVOI BM (2018) Light and scanning electron microscopy of the olfactory mucosa in the rufous sengi (Elephantulus rufescens). Anat Histol Embryol, 47:167-173.
KAVOI BM, JAMEELA H (2011) Comparative morphometry of the olfactory bulb, tract and stria in the human, dog and goat. Int J Morphol, 29: 939-946.
KAVOI BM, KISIPAN ML (2019) A gross morphometric study of olfactory brain components in the Rufous Sengi (Elephantulus rufescens). Int J Morphol, 37: 1003-1007.
KAVOI B, MAKANYA A, HASSANALI J, CARLSSON HE, KIAMA S (2010) Comparative functional structure of the olfactory mucosa in the domestic dog and sheep. Ann Anat, 192: 329-337.
KAVOI BM, MAKANYA AN, PLENDL J, KIAMA SG (2012) Morphofunctional adaptations of the olfactory mucosa in postnatally developing rabbits. Anat Rec, 295: 1352-1363.
MATTHEWS J (2008) The Value of Grasscutters. World Ark, (January-February, 2008), pp 23-24.
MOTA-ROJAS D, ORIHUELA A, NAPOLITANO F, MORA-MEDINA P, OROZCO-GREGORIO H, ALONSO-SPILSBURY M (2018) Invited review: Olfaction in animal behaviour and welfare. CAB Rev, 13: 1-13.
ONYONO PN, KAVOI BM, KIAMA SG, MAKANYA AN (2017) Functional morphology of the olfactory mucosa and olfactory bulb in fossorial rodents: The East African root rat (Tachyoryctes splendens) and the naked mole rat (Heterocephalus glaber). Tissue Cell, 49: 612-621.
OPARA MN (2010) The Grasscutter I: A livestock of tomorrow. Res J For, 4: 119-135.
PIHLSTRÖM H, FORTELIUS M, HEMILÄ S, FORSMAN R, REUTER T (2005) Scaling of mammalian ethmoid bones can predict olfactory organ size and performance. Proc R Soc B, 272: 957-962.
RAIMUND A, DIETER R, BURTON MS (1991) Ontogenetic changes in odor sensitivity, olfactory receptor area and olfactory receptor density in the rat. Chem Senses, 16: 209-218.
ROSEVEAR DR (1969) The Rodents of West Africa. British Museum Publications, London.
SAKASHITA H, MORIIZUMI T, ITO M, FURUKAWA M, KAWANO J OKOYAMA S, KITAO Y, KUDO M (1995) Differentiation of the olfactory epithelium during development. Acta Otorrinolaringol, 115: 93-98.
SCHERLE W (1970) A simple method for volumetry of organs in quantitative stereology. Mikroskopie, 26: 57-60.
VAN DRONGELEN W, HOLLEY A, DOVING KB (1978) Convergence in the olfactory system: Quantitative aspects of odor sensitivity. J Theor Biol, 71: 39-48.
WILLIAMS OS, OLA SI, BOLADURO BA, BADMUS RT (2011) Diurnal variation in ambient temperature and humidity in a pit pen grass-cutter (Thryonomys swinderianus) house in Ile-Ife. Proceedings of 36th Annual Conference Nigerian Society for Animal Production. Abuja, Nigeria, pp 111-113.
WOODS CA, KILPATRICK CW (2005) Infraorder Hystricognathi Brandt, 1855. In: Wilson DE, Reeder DM (eds). Mammal Species of the World: a Taxonomic and Geographic Reference, 3rd ed. The Johns Hopkins University Press, Baltimore, pp 1538-1600.