The aim of this study is to establish age and sex based-reference values of corpus callosum (CC) length and its distancesto the fornix and anterior commissure. Brain MRIs of 214 subjects, who had normal MRI scans according to radiologists’reports, were reviewed retrospectively. Scans were obtained from April 2013 until April 2014 at Jordan UniversityHospital, Radiology Department (Amman, Jordan). The antero-posterior (longitudinal) dimensions of the CC (AB), the vertical distance between the anterior commissure and the most superior point of CC (CD), the distance between the genuand the fornix (AE), and the angle between AB (the longitudinal dimension of the CC) and AD (the distance from the genu to the anterior edge of the anterior commissure) (BAD) were measured and subjected to statistical analysis.Regardless of sex, CC length and other parameters (CD and AE) increased dramatically with age in children andadolescents (≤ 18years). A significant negative correlation was found between age and BAD angle in females only. Weobserved a continuous increase in CC length after childhood in both sexes, with a slower rate of growth in females.Sexual dimorphism was observed in AB and BAD angle in infants (≤ 2years) and AE in the adolescent group (12 to 18years old). A local reference of CC length and its distances to nearby structures were established. Sexual dimorphisms of some CC parameters were more evident during the early years of life. Our data also indicated important sex differences in the development of the CC. Such knowledge is essential in evaluating the normal neurological development of the CC and the pathological conditions affecting it.
Gender and age-related differences in the morphometry of corpus callosum: MRI study
Maher T. Al-Hadidi1, Heba M. Kalbouneh2, Ashraf Ramzy3, Aiman Al Sharei3, Darwish H. Badran2,3, Amjad Shatarat2, Emad S. Tarawneh4, Waleed S. Mahafza4, Fadi A. Al-Hadidi5, Azmy M. Hadidy3
1 Department of Anatomy and Histology, Faculty of Medicine, Al-Balqa Applied University, Al Salt, Jordan.
2 Department of Anatomy and Histology, School of Medicine, The University of Jordan, Al Salt, Jordan.
3 Department of Basic Medical Sciences, School of Medicine, The Hashemite University, Zarqa, Jordan.
4 Department of Diagnostic Radiology and Nuclear Medicine, School of Medicine, The University of Jordan, Amman, Jordan.
5 Department of Special Surgery, School of Medicine, The University of Jordan, Amman, Jordan
SUMMARY
Sign up or Login
Introduction
The corpus callosum (CC) is the largest white matter structure in the human brain. It connects the right and left cerebral hemispheres in a mostly homotopic fashion. The CC lies in the midsagittal plane between the two hemispheres with the fornix and the third ventricle related to it inferiorly (Griffiths et al., 2009). The growth and shape of the CC are determined by several factors including age, prematurity, genetics, handedness, childhood neglect, and gender (Garel et al., 2011; Nosarti et al., 2004; Teicher et al., 2004; Westerhausen et al., 2004; Woldehawariat et al., 2014).
Neurologists can use CC dimensions and its relation to the nearby structures to compare with certain neurological and psychological disease entities (Luders et al., 2010; Woldehawariat et al., 2014). Most of the studies on the size and shape of CC were performed outside the Middle East and other countries to confirm the genetic and racial factors in anthropometry. In our study population, the mean CC length was similar to the data recorded from Iranian and Turkish populations (Mohammadi et al., 2011; Unlu et al., 2014), but was higher than the data from the Japanese (Takeda et al., 2003), indicating that ethnicity could play an important role in CC size (Mohammadi et al., 2011).
Alterations in the size and morphology of the CC are commonly documented in several psychiatric disorders. For example, alterations in the size and shape of the CC were reported in patients with schizophrenia and bipolar disorder (Downhill et al., 2000; Walterfang et al., 2009). Furthermore, schizophrenia patients had reduced CC total area and length when compared with controls (Unlu et al., 2014). Fractional anisotropy (a diffusion tensor imaging measure that reflects the degree to which white matter fibers are aligned in a specific direction) of the genu, splenium and total corpus callosum was significantly reduced in autism (Alexander et al., 2007). Additional studies provide preliminary evidence of impaired neural connectivity in the CC and other major fiber tracts in autism (Barnea-Goraly et al., 2004; Jou et al., 2011). A reduction in the CC area was also found to be associated with impulsivity, as well as suicidal behavior (Cyprien et al., 2011; Moeller et al., 2005). Thus, CC morphometric data may indirectly reflect underlying pathologic processes. In this study, our aim is to investigate the possible sex-related differences in the length of the CC, its morphometric relationship to nearby structures (the fornix and anterior commissure) and the postnatal developmental changes based on cross-sectional MRI data from healthy Jordanian children and adults.
Material and methods
Cerebral MRIs from the midsagittal plane were collected from April 2013 until April 2014 at Jordan University Hospital, Radiology Department (Amman, Jordan). Informed consent was obtained from all study participants or their legal guardians, and ethical approval was obtained from the Academic Research Council of the Faculty of Medicine, University of Jordan, according to the ethical principles of the Declaration of Helsinki.
Two hundred and fourteen normal MRIs were obtained from 95 males and 119 females (mean age ± SD: 27.0±21.8 and 27.9±19.5 years, respectively). The subjects of the sample were selected from those referred for advanced evaluation of the brain by MRI and proved to be negative. Only normal MRI scans (according to the radiologists’ reports) were included.
Exclusion criteria
For our study, the following exclusion criteria were used: a) intracranial lesions, masses or head injury on the MRI, b) demyelination and degenerative diseases (multiple sclerosis), c) history of neurosurgery, d) previous cerebrovascular accidents, e) history of neurological or psychiatric diseases (epilepsy, autism, depression, dementia, schizophrenia and bipolar disorder), and g) CC anomalies (hypoplasia, dysplasia, and complete agenesis). None of the participants had ever received psychopharmacotherapy.
Using 18 years as the beginning of adulthood, the subjects were divided into two main groups: group 1) children and adolescents (≤ 18 years), and group 2) adults (>18 years). To study the developmental sexual dimorphism of the CC, each of these two groups was further subdivided into subgroups on the basis of age as follows: infancy (birth to 2 years old), early childhood (3-8 years old), middle childhood (9-11 years old), adolescence (12-18 years old), young adulthood (19-39 years old), middle adulthood (40-60 years old), and late adulthood (>60 years old).
Procedure
A Siemens Magnetic Vision Plus (1.5 Tesla) (Siemens, Erlangen, Germany) was used in T1 sequence (TR=600, TE=14) with a 512x512 matrix and 32 cm FOV. Twelve sagittal images scanning from right to left were performed in two minutes for each patient while in a supine position. The sagittal images were parallel to the axial plane of the CC. The obtained images were reconstructed at 5 mm slices with a verified inclination of less than 1° from the midline. The best image showing the CC was selected. The parameters were measured by Syngo Fast View software (Siemens Medical Solutions, Department SW, Erlangen, Germany) on the same slice image and recorded to the nearest millimeter. The distances were measured directly from the MRI screen and documented on printed images. The anteroposterior length of the corpus callosum (AB) was taken as the distance between the anterior edge of the genu and the posterior edge of the splenium. The distance from the anterior edge of genu to the anterior edge of the fornix (AE) was measured, and the anterior edge of its column was considered at the bifurcation above the anterior commissure. The maximum height (CD) of the CC was taken as the vertical distance from the anterior commissure to the dorsal edge of the CC. The angle (BAD) formed by the meeting of the longitudinal dimension of CC (AB) and the distance from the genu to the anterior edge of the anterior commissure (AD) was also measured (Fig. 1).
Three senior radiologists (each with at least 10 years of experience in brain MRI) took all of the measurements (three values for each parameter) and the combined mean values were used in the subsequent analysis of each data set.
Statistical analysis
The data was entered into a spreadsheet and analyzed using the IBM SPSS Statistics for Windows, version 19 (IBM Corp, Armonk, NY, USA). The means (± standard deviation) were calculated, and males and females in the various age groups were compared.
Differences in continuous variables between the two independent groups were assessed using the two-tailed t test. Relationships between each dimension and the ages were assessed using the Spearman’s Rho coefficient. The significance threshold was set at 0.05. All measurements showed excellent interobserver reliability, with intraclass correlation coefficients ranging from 0.76 to 0.83. The XY scatter plots were generated using Microsoft Excel 2010 and the figures were generated using Adobe Illustrator CC 2014.
Results
CC development from childhood to adulthood
MRIs from children and adults who were 18 years of age or younger were examined separately for age-related changes in CC length, and its morphometric relationship to the fornix and anterior commissure was also examined.
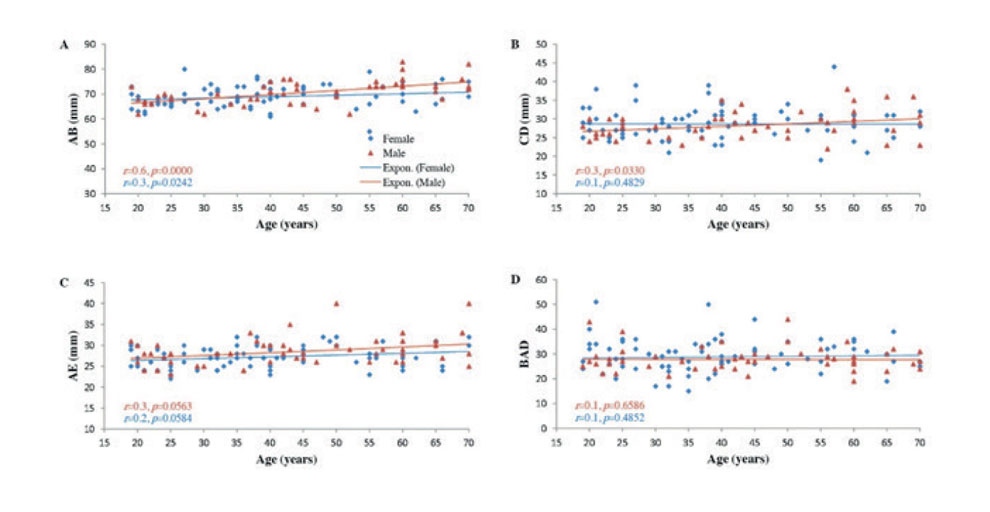
In males, the Spearman’s Rho coefficient between age and CC parameters showed a positive relationship in children and adolescents (≤ 18 years). A very strong positive correlation was evident with AB (r = 0.8), a moderate positive correlation was evident with CD (r = 0.5), and a very strong positive correlation was evident with AE (r = 0.8). These correlations were statistically significant (p<0.001). A very weak negative correlation between age and BAD angle was also observed (r = -0.1), but it was not statistically significant (p>0.05) (Fig. 2).
In female children and adolescents (≤ 18 years), a strong positive correlation was noted with AB (r = 0.8), and moderate positive correlations were evident with CD (r = 0.33) and AE (r = 0.5). However, the correlation between age and BAD angle was much stronger in females than males, and a moderate negative correlation was noted (r = -0.4) (Fig. 2). All correlations were statistically significant (p<0.05).
In adult males (>18 years), a moderate positive correlation was evident with AB (r = 0.6). Weaker positive correlations were evident with CD and AE (r = 0.3), and a statistically insignificant positive correlation was observed with BAD angle (r = 0.1) (Fig. 3).
In adult females (>18 years), a weak positive correlation that was still statistically significant was noted between age and AB (r = 0.3, p<0.05). Weak positive correlations that were statistically insignificant were noted between age and both CD (r = 0.1) and AE (r = 0.2) (p>0.05). A statistically insignificant, weak positive correlation was noted between age and BAD angle (r = 0.1) (Fig. 3).
Gender and age-related differences in CC length and its morphometric relationship to the fornix and anterior commissure
The mean values (± SD) of CC length and distances to the fornix and anterior commissure in males and females of the different age subgroups are shown in Table 1. For most of the CC parameters, males tended to have higher mean values than females. In contrast, the BAD angle was higher in females. A comparison of mean CC parameters between males and females (using the two-tailed t-test) showed a statistically significant difference (P<0.05) in the longitudinal dimension of the CC (AB) and the BAD angle in only the infant subgroups (birth - 2 years old). The distance between the genu and the fornix (AE) was also significantly different between genders in the adolescent group (12-18 years old).
Discussion
CC dimensions and its distances to the nearby structures can be used by neurologists to compare the findings in patients with certain neurological and psychological disease entities (Luders et al., 2010; Woldehawariat et al., 2014). Most of the studies on the size and shape of CC were performed on Caucasian (Mourgela et al., 2007), East Asian (Hwang et al., 2004), Iranian (Mohammadi et al., 2011) and Indian populations (Gupta et al., 2008). Few studies have been performed using Middle Eastern individuals. In a previous study, Yasin and Farahani (2015) reported that genetic and racial factors, in addition to educational background, play a significant role in CC size. The authors suggested that such research should be conducted in the Middle East and other countries to confirm the genetic and racial factors in anthropometry. In our study population, the mean CC length was similar to the data recorded from Iranian and Turkish populations (Mohammadi et al., 2011; Unlu et al., 2014). Our values, however, still higher than the data from the Japanese (Takeda et al., 2003), indicating that ethnicity could play an important role in CC size (Mohammadi et al., 2011).
Currently, many factors are thought to influence the variations observed in human CC morphology. The first factor considered in our study was the age of the participants. According to 3D ultrasounds of the fetal CC, rudimentary fibers of the CC begin to appear at the fetal midline in the 12th week of gestation (Pashaj et al., 2013). The number of callosal fibers is already fixed around birth, but structural changes continue to occur in the CC during postnatal development (Luders et al., 2010; Tanaka-Arakawa et al., 2015). Our data showed a rapid increase in CC length until 18 years, but slower growth in adults of both sexes (>18 years).
Numerous studies have reported that CC length changes significantly with age (Luders et al., 2010; Mohammadi et al., 2011), and our findings confirm this observation. However, few studies have reported data on CC morphology in infants. A study of healthy children with ages ranging from 1 day to 15 years reported rapid growth until three years of age, followed by continuous slower growth (Garel et al., 2011). In the study by Garel et al., the fronto-occipital diameter (anteroposterior diameter of the CC) and the thickness of the genu and isthmus were measured, but they did not directly assess the size of the CC. On the other hand, a recent MRI study of 114 healthy individuals with ages ranging from 1 month to 25 years reported significant, non-linear, age-related increases in the absolute area of the overall CC and its subregions: rostrum, genu, rostral body, anterior midbody, posterior midbody, isthmus and splenium. The growth rate then started to flatten during adolescence and adulthood in both sexes (Tanaka- Arakawa et al., 2015). Our results echo their findings, but we further showed a continued increase in CC length after the childhood period in both sexes, with a greater slowing of growth in females. Using MRI, Vannucci et al. (2017) reported that genu, body, splenial and total corpus callosal areas increased by 40-100% during first year of life. Comparing genu and splenium, they demonstrated that the genu expanded to a greater extent than the splenium during the first 6 years, while the splenium expanded to a greater extent between 7 and 18 years. They attributed these age-related differences to the consequence of differential axonal myelination. Sex differences seem to exist during the development of the CC. The significant increase in the CC parameters in males explains the significant increase of the BAD angle in females.
Geometrically, the meeting of the AD line with a longer AB line (at A point) produces a more acute angle than the meeting of the same AD line with a shorter AB line (at a closer A point), which will produce a wider angle. Our results support the idea that structural changes of the CC continue to occur throughout life, but the most dramatic changes happen during childhood and adolescence. Progressive fiber myelination, regressive pruning processes, glial proliferation and ongoing maturation and modeling of the axonal cytoskeleton may underlie these changes (Luders et al., 2010). The adult group in the present study ranged in age from 19-70 years and a wider age range of participants could have yielded different results. Westerhausen et al. (2011) suggested sexual dimorphism in the micro-structural architecture of the CC. They claimed that clusters of significantly-higher fractional anisotropy and lower diffusion strength in males compared to females were detected in the genu and truncus of CC.
The literature concerning sexual dimorphism of the CC is not in agreement about sex differences in callosal size and shape (Ardekani et al., 2013; Gupta et al., 2008; Luders et al., 2010). In one study, the only parameter that showed significant gender dimorphism was the length of the CC, being longer in males (Suganthy et al., 2003). In Iran and Japan, however, no statistically significant differences between the sexes were found (Mohammadi et al., 2011; Takeda et al., 2003). In our study, the male to female differences in the longitudinal dimensions of the CC were not statistically significant. Of the parameters measured, only the longitudinal dimension of the CC and BAD angle in infants (≤ 2 years) and the distance between the genu and the fornix (AE) in adolescents (12-18 years old) exhibited sexual dimorphism. This may be explained by a significant difference in the growth of the CC parameters in adolescents between sexes, which may be attributed to hormonal factors. Guz et al. (2019) studied sexual parameters as regards CC including: lengths of longitudinal cross-section of CC, CC thickness in the narrowest place (isthmus), the largest linear dimension of the brain from the frontal pole to occipital pole, the longitudinal cross-section area of the CC and cerebral cross-section area. They demonstrated that in all age groups studied, there were statistically-significant differences in the values of the analysed parameters and ratios of CC size between males and females.
Several studies have reported the presence of significant sex differences in callosal measurements. DeLacoste-Utamsing and Holloway (1982) reported a sex difference in the shape and surface area of the CC, especially in its splenium. They related such observation due to gender differences in the degree of lateralization for visuospatial function, since the splenium contains peristriate, parietal and superior temporal fibers. In Korean neonates, Hwang et al. (2004) reported no significant gender differences in the width and total area of the CC, but the height of the CC was greater in males and the splenium was thicker in females. Similar results were also obtained in a Turkish population where the splenium was found to be wider in females than in males (Aydlnlioglu et al., 1996). In an Indian study, morphometric measurements of the CC and its subregions showed sexual dimorphism in several parameters: 1) a longer CC in males, 2) a longer distance between the genu and the fornix in older males, and 3) a longer distance between the splenium and superior colliculus (Gupta et al., 2008). Another earlier study reported significantly higher ratios between the whole brain and the total CC including the genu, the posterior midbody, and the splenium in females than males (Tanaka-Arakawa et al., 2015).
Little is yet known about the morphometric relationship between CC and nearby structures. In our study, the distances from the CC to the fornix and anterior commissure (CD and AE respectively) were also traced from infancy to late adulthood. In both sexes, CD and AE exhibited significant increases with advancing age until 18 years. However, the angle formed by the longitudinal dimension of the CC and the distance from the genu to the anterior edge of the anterior commissure (BAD) decreased significantly with age in female children and adolescents only. In adulthood, the CD continued to increase significantly with age in males only. These data suggest age-related sex differences during the structural maturation of the brain. Myelination processes could also affect the anatomical relationship between the CC and the nearby structures. Additionally, gonadal hormones and sex chromosomes could contribute to differences between the sexes. Schmied et al. (2020) studied sex differences in brain-size adjusted CC area and thickness in ages of 6-24 months and reported a steeper rate of growth in males versus females. They reported that CC thickness was significantly associated with underlying microstructural organization. However, they observed that there were no sex differences in the association between microstructure and thickness, suggesting that the role of factors such as axon density and/or myelination in determining CC size is generally equivalent between sexes.
Our study was limited, because we did not measure the thickness of the CC and its subregions. We only measured the length of the CC, as it is easily applicable in daily clinical practice. However, more detailed analyses of the callosal subregions should be conducted to establish a normative reference in our population and to understand the complex changes in CC morphometry throughout life. In addition, our results only reveal differences between people of different ages rather than changes in the same individuals over time. Therefore, a longitudinal study of these changes could provide more insight into developmental changes in the CC.
In conclusion, this study offers a reference of CC length, and its distances from the anterior commissure and fornix are established. In addition, sexual dimorphisms of some CC parameters were more evident during the early years of life. Our data also indicated important sex differences in the development of the CC. Such knowledge is essential in evaluating the normal neurological development of the CC and the pathologies affecting it. Further studies with increased numbers of samples can give more evidence concerning these parameters.
Related articles
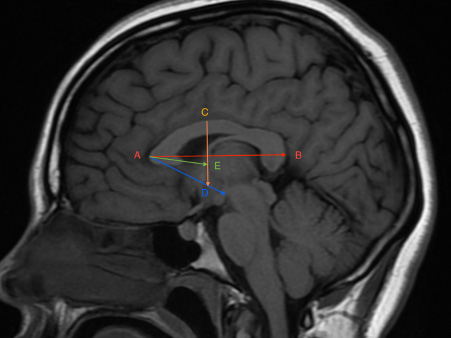 Fig. 1.- MRI showing the anteroposterior length measurement of the corpus callosum and its relation to the fornix and anterior commissure. AB: The length of the corpus callosum from the anterior edge of the genu to the posterior edge of the splenium. AE: The distance from the anterior edge of genu to the anterior edge of the fornix at its bifurcation above the anterior commissure. CD: The maximum height from the anterior commissure to the dorsal edge of the CC. BAD: The angle formed by the meeting of AB and AD (the distance from the genu to the anterior edge of the anterior commissure).
Fig. 1.- MRI showing the anteroposterior length measurement of the corpus callosum and its relation to the fornix and anterior commissure. AB: The length of the corpus callosum from the anterior edge of the genu to the posterior edge of the splenium. AE: The distance from the anterior edge of genu to the anterior edge of the fornix at its bifurcation above the anterior commissure. CD: The maximum height from the anterior commissure to the dorsal edge of the CC. BAD: The angle formed by the meeting of AB and AD (the distance from the genu to the anterior edge of the anterior commissure).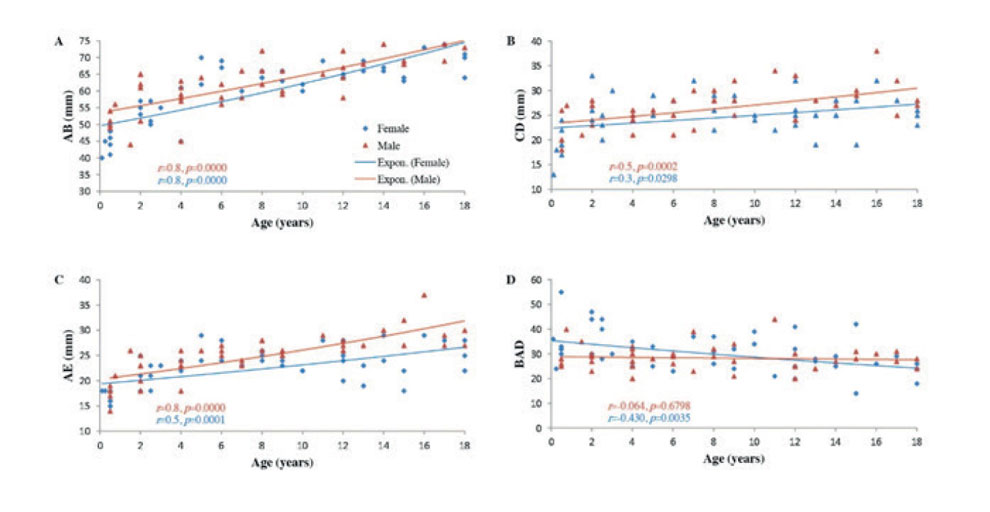 Fig. 2.- Scatter plots showing the correlations between age and: A: AB, B: CD, C: AE, and D: BAD angle in childhood and adolescence (0-18 years). Regression lines represent the lines of best fit. r: Spearman's Rho coefficient. P values were calculated using the two-tailed t-test, n=88.
Fig. 2.- Scatter plots showing the correlations between age and: A: AB, B: CD, C: AE, and D: BAD angle in childhood and adolescence (0-18 years). Regression lines represent the lines of best fit. r: Spearman's Rho coefficient. P values were calculated using the two-tailed t-test, n=88.ALEXANDER AL, LEE JE, LAZAR M, BOUDOS R, DUBRAY MB, OAKES TR, MILLER JN, LUJ, JEONG EK, MCMAHON WM, BIGLER ED, LAINHART JE (2007) Diffusion tensor imaging of the corpus callosum in autism. Neuroimage, 34(1): 61-73.
ARDEKANI BA, FIGARSKY K, SIDTIS JJ (2013) Sexual dimorphism in the human corpus callosum: an MRI study using the OASIS brain database. Cereb Cortex, 23(10): 2514-2520.
AYDLNLIOGLU A, DIYARBAKIRLI S, YÜCEER N, KELES P, UNAL O, ERDOGAN RA (1996) The relationship of sex differences to the anatomy of corpus callosum in the living human being. Turkish Neurosurgery, 6: 1-4.
BARNEA-GORALY N, KWON H, MENON V, ELIEZ S, LOTSPEICH L, REISS AL (2004) White matter structure in autism: preliminary evidence from diffusion tensor imaging. Biol Psychiatry, 55(3): 323-326.
CYPRIEN F, COURTET P, MALAFOSSE A, MALLER J, MESLIN C, BONAFE A, LE BARS E, DE CHAMPFLEUR NM, RITCHIE K, ARTERO S (2011) Suicidal behavior is associated with reduced corpus callosum area. Biol Psychiatry, 70(4): 320-326.
DELACOSTE-UTAMSING C, HOLLOWAY RL (1982) Sexual dimorphism in the human corpus callosum. Science, 216(4553): 1431-1432.
DOWNHILL JE JR, BUCHSBAUM MS, WEI T, SPIEGEL-COHEN J, HAZLETT EA, HAZNEDAR MM, SILVERMAN J, SIEVER LJ (2000) Shape and size of the corpus callosum in schizophrenia and schizotypal personality disorder. Schizophr Res, 42(3): 193-208.
GAREL C, CONT I, ALBERTI C, JOSSERAND E, MOUTARD ML, DUCOU LE POINTE H (2011) Biometry of the corpus callosum in children: MR imaging reference data. AJNR Am J Neuroradiol, 32(8): 1436-1443.
GRIFFITHS PD, BATTY R, REEVES MJ, CONNOLLY DJ (2009) Imaging the corpus callosum, septum pellucidum and fornix in children: normal anatomy and variations of normality. Neuroradiology, 51(5): 337-345.
GUPTA T, SINGH B, KAPOOR K, GUPTA M, KOCHHAR S (2008) Age and sex related variations in corpus callosal morphology. Nepal Med Coll J, 10(4): 215-221.
GUZ W, PAZDAN D, STACHYRA S, SWIETON F, PORĘBA P, BEDNARZ M, LIS A, KAZAŃSKA A, KRUKOWSKA J, KLĘBA J, URBANIK A (2019) Analysis of corpus callosum size depending on age and sex. Folia Morphol, 78(1): 24-32.
HWANG SJ, JI EK, LEE EK, KIM YM, SHIN DY, CHEON YH, RHYU IJ (2004) Gender differences in the corpus callosum of neonates. Neuroreport, 15(6): 1029-1032.
JOU RJ, JACKOWSKI AP, PAPADEMETRIS X, RAJEEVAN N, STAIB LH, VOLKMAR FR (2011) Diffusion tensor imaging in autism spectrum disorders: preliminary evidence of abnormal neural connectivity. Aust N Z J Psychiatry, 45(2): 153-162.
LUDERS E, THOMPSON PM, TOGA AW (2010) The development of the corpus callosum in the healthy human brain. J Neurosci, 30(33): 10985-10990.
MOELLER FG, HASAN KM, STEINBERG JL, KRAMER LA, DOUGHERTY DM, SANTOS RM, VALDES I, SWANN AC, BARRATT ES, NARAYANA PA (2005) Reduced anterior corpus callosum white matter integrity is related to increased impulsivity and reduced discriminability in cocaine-dependent subjects: diffusion tensor imaging. Neuropsychopharmacology, 30(3): 610-617.
MOHAMMADI MR, ZHAND P, MORTAZAVI MOGHADAM B, GOLALIPOUR MJ (2011) Measurement of the corpus callosum using magnetic resonance imaging in the north of iran. Iran J Radiol, 8(4): 218-223.
MOURGELA S, ANAGNOSTOPOULOU S, SAKELLAROPOULOS A, GOULIAMOS A (2007) An MRI study of sex- and age-related differences in the dimensions of the corpus callosum and brain. Neuroanatomy, 6(1): 63-65.
NOSARTI C, RUSHE TM, WOODRUFF PW, STEWART AL, RIFKIN L, MURRAY RM (2004) Corpus callosum size and very preterm birth: relationship to neuropsychological outcome. Brain, 127(Pt 9): 2080-2089.
PASHAJ S, MERZ E, WELLEK S (2013) Biometry of the fetal corpus callosum by three-dimensional ultrasound. Ultrasound Obstet. Gynecol, 42(6): 691-698.
SCHMIED A, SODA S, GERIG G, STYNER M, SWANSON MR, ELISON JT, SHEN MD, MCKINSTRY RC, PRUETT JR JR, BOTTERON KN, ESTES AM, DAGER SR, HAZLETT HC, SCHULTZ RT, PIVEN J, WOLFF JJ (2020) Sex differences associated with corpus callosum development in human infants: A longitudinal multimodal imaging study. Neuroimage, 215: 116821.
SUGANTHY J, RAGHURAM L, ANTONISAMY B, VETTIVEL S, MADHAVI C, KOSHI R (2003) Gender- and age-related differences in the morphology of the corpus callosum. Clin Anat, 16(5): 396-403.
TAKEDA S, HIRASHIMA Y, IKEDA H, YAMAMOTO H, SUGINO M, ENDO S (2003) Determination of indices of the corpus callosum associated with normal aging in Japanese individuals. Neuroradiology, 45(8): 513-518.
TANAKA-ARAKAWA MM, MATSUI M, TANAKA C, UEMATSU A, UDA S, MIURA K, SAKAI T, NOGUCHI K (2015) Developmental changes in the corpus callosum from infancy to early adulthood: a structural magnetic resonance imaging study. PLoS One, 10(3): e0118760.
TEICHER MH, DUMONT NL, ITO Y, VAITUZIS C, GIEDD JN, ANDERSEN SL (2004) Childhood neglect is associated with reduced corpus callosum area. Biol Psychiatry, 56(2): 80-85.
UNLU E, BAGCIOGLU E, ACAY MB, KACAR E, TURAMANLAR O, GONUL Y, CEVIK M, AKPINAR A, COSKUN KS (2014) Magnetic resonance imaging study of corpus callosum abnormalities in patients with different subtypes of schizophrenia. S Afr J Psychiatr, 20(4): 146-152.
VANNUCCI RC, BARRON TF, VANNUCCI SJ (2017) Development of the corpus callosum: an MRI study. Dev Neuroscience, 39(1-4): 97-106.
WALTERFANG M, WOOD AG, BARTON S, VELAKOULIS D, CHEN J, REUTENS DC, KEMPTON MJ, HALDANE M, PANTELIS C, FRANGOU S (2009) Corpus callosum size and shape alterations in individuals with bipolar disorder and their first-degree relatives. Prog Neuropsychopharmacol Biol Psychiatry, 33(6): 1050-1057.
WESTERHAUSEN R, KOMPUS K, DRAMSDAHL M, FALKENBERG LE, GRÜNER R, HJELMERVIK H, SPECHT K, PLESSEN K, HUGDAHL K (2011) A critical re-examination of sexual dimorphism in the corpus callosum microstructure. Neuroimage, 56(3): 874-880.
WESTERHAUSEN R, KREUDER F, DOS SANTOS SEQUEIRA S, WALTER C, WOERNER W, WITTLING RA, SCHWEIGER E, WITTLING W (2004) Effects of handedness and gender on macro- and microstructure of the corpus callosum and its subregions: a combined high-resolution and diffusion-tensor MRI study. Brain Res Cogn Brain Res, 21(3): 418-426.
WOLDEHAWARIAT G, MARTINEZ PE, HAUSER P, HOOVER DM, DREVETS WW, MCMAHON FJ (2014) Corpus callosum size is highly heritable in humans, and may reflect distinct genetic influences on ventral and rostral regions. PLoS One, 9(6): e99980.
YASIN M, FARAHANI RM (2015) Corpus callosum size, is there a sexual difference. Int J Morphol, 33(1): 96-99.