Nasal polyps are outgrowths of oedematous nasal mucosa of inflammatory origin. Langerhans cells (LCs) residing in the nasal mucosa are responsible for maintaining the immunological environment. This study aims to ascertain the distribution of zinc iodide osmium (ZIO) positive LCs in normal nasal mucosa, and to compare the same with that in polypoid mucosa. Normal nasal mucosa obtained from patients undergoing septoplasty (n=21) and polypoid mucosa (n=21) obtained from patients with sinonasal polyposis due to diffuse sinonasal inflammation or allergic fungal rhinosinusitis were processed for identification of LCs using the ZIO technique. The number of ZIO positive LCs were counted per unit area of lamina propria and statistically analysed. The ZIO positive LCs were seen in the epithelium, lamina propria and among the glands. Both dendritic and non-dendritic LCs were present. They were noted within the lymphatic aggregations in the lamina propria, and were also noted in and around the blood vessels. The ZIO positive LCs were present in both normal and polypoid nasal mucosa. The median number of ZIO positive LCs was 0 (range: 0-1) per mm2 and 12 (range: 0-44) per mm2 in the normal and polypoid groups, respectively. This difference was statistically significant (p=0.001). No significant difference was noted based on the aetiology of the polyps. This is the first report of the presence of ZIO positive LCs in the nasal mucosa. Their significantly higher number in nasal polyps suggests an immunological role in the presence of inflammation.
Evidence of ZIO positive Langerhans cell: a dendritic cell subset in normal and polypoid nasal mucosa
J. Rachel1, John K. Kulathu Mathew1, Sam Marconi D3, Zorem Sangi2, Vedantam Rupa2, Suganthy Rabi1
1 Department of Anatomy, Christian Medical college, Vellore-632002, India
2 Department of Otorhinolaryngology, Christian Medical college, Vellore-632002, India
3 Department of Community Health and Development, Christian Medical college, Vellore-632002, India
SUMMARY
Sign up or Login
INTRODUCTION
The nasal cavity is lined with both the respiratory and olfactory epithelium. The respiratory epithelium is made up of pseudostratified ciliated columnar epithelium lining most surfaces of the nasal cavity. This barrier of the immune system provides mechanical protection from infectious and allergenic pathogens (Orahilly et al., 1967). A nasal polyp is a protrusion of oedematous nasal mucosa that occurs secondary to underlying inflammation. Histologically, polyps consist of a layer of pseudostratified columnar epithelium with several mucus secreting goblet cells and an underlying oedematous stroma with sparse blood vessels, fibrous tissue and nerves. In allergic polyps a large number of eosinophils may be seen in the lamina propria. The aetiology of sinonasal polyposis is varied; allergy, infection, allergic fungal rhinosinusitis, vasomotor rhinopathy, aspirin sensitivity are some common conditions associated with polyposis (Önerci, 2010). Allergic fungal sinusitis is a type 1 hypersensitivity reaction to a fungal allergen characterised by the presence of diffuse sinonasal polyposis and allergic mucin.
Human dendritic cells are the most potent antigen presenting cells, crucially linking the innate and adaptive immune responses (Adema, 2009; Schroeder et al., 2005). Their presence at the body’s portals of entry for antigens, aids in fulfilling their function of antigen processing. At the mucosal surface, a distinct subset called the Langerhans cells (LCs) plays a major role in humoral and cell-mediated immunity. Various histochemical techniques employed to demonstrate LCs include gold chloride, zinc iodide osmium (ZIO) and uranyl acetate-lead staining (Jaitley and Saraswathi, 2012). Although the role of LCs in infections like rhinosinusitis (Pezato et al., 2014) have previously been studied using various markers, the ZIO positive LCs were not demonstrated in nasal mucosa. Therefore, the present study aimed to demonstrate the morphology and distribution of ZIO positive LCs in normal as well as polypoid nasal mucosa.
MATERIALS AND METHODS
Institutional Review Board and Ethics Committee approval was obtained for conducting the study (IRB Min:10895). Two groups of patients that were seen in the department of Otorhinolaryngology were recruited to the study. The first group (Group I) consisted of 21 patients with no evidence of polyposis, and who had otherwise normal- appearing mucosa. These patients were being operated by septoplasty for a deviated nasal septum. A small piece of redundant normal septal mucosa was resected in these patients. In the second group (Group II), 21 adult patients who underwent functional endoscopic sinus surgery for sinonasal polyposis during the study period were recruited to the study. This group included patients with diffuse sinonasal polyposis of allergic or other aetiology, as well as cases of allergic fungal sinusitis. Informed consent was obtained from all the patients who participated in the study. Patients with immunocompromised status, history of chemotherapy/radiation and invasive infections or neoplasms were excluded.
The polyps and normal nasal mucosal tissue were collected in an icebox and immediately fixed in veronal-buffered zinc iodide-osmium tetroxide, pH 7.4 (Figueroa and Caorsi, 1980) for 72 hours at 4°C in the dark, washed in distilled water, dehydrated in graded ethanol, cleared in xylene and embedded in paraffin wax. Serial sections of 6 µm thickness were taken. The sections were transferred to glass slides, deparaffinized, mounted using DPX and viewed under a microscope.
The slides were studied under the Olympus BX43 microscope with an inbuilt Olympus DP21 camera, and the software used was CellSens image analysing software (version 1.4.). The number of ZIO positive LCs were counted under 40X magnification using the closed polygon-measuring option of the image-analysing software over 20 fields. Each field measured ~50,000 μm2, thus making a total area of 1 mm2. Data analysis was done using SPSS version 24.
RESULTS
The age distribution of patients in both groups was similar. The age of patients in group I ranged from 23 to 62 years (mean = 40.5 ± 10.79), and in group II it ranged from 18 to 65 years (mean = 40.6 ± 11.4). There were 13 male and 8 female patients in group I and 7 male and 14 female patients in group II.
LCs in nasal mucosa of group I patients
The nasal mucosa was lined by pseudostratified ciliated columnar epithelium in most of the patients, and the ZIO positive LCs were nearly absent in the surface epithelium (Fig. 1a). In patients who had hyperplastic pseudostratified ciliated columnar epithelium, few non-dendritic ZIO positive LCs were noted (Fig. 1b). A few patients had squamous metaplasia in which no ZIO positive LCs were seen. Lamina propria had connective tissue with mucosal glands, blood vessels and nerve fibres. The ZIO positive LCs were sparsely seen within glands (Fig. 1a). The number of ZIO positive LCs ranged from 0 to 4 (mean = 0.67 ± 1.238 per mm2) (Table 1).
Table 1. Number of ZIO positive LCs in both groups per mm2 area of lamina propria (n=21).
|
Range |
Mean (SD) |
Median |
IQR |
p-value |
|
|
Group I |
0-4 |
0.67±1.23 |
0 |
1 |
0.001 |
|
Group II |
0-97 |
24.38 ± 29.54 |
12.0 |
44 |
LCs in nasal mucosa of group II patients
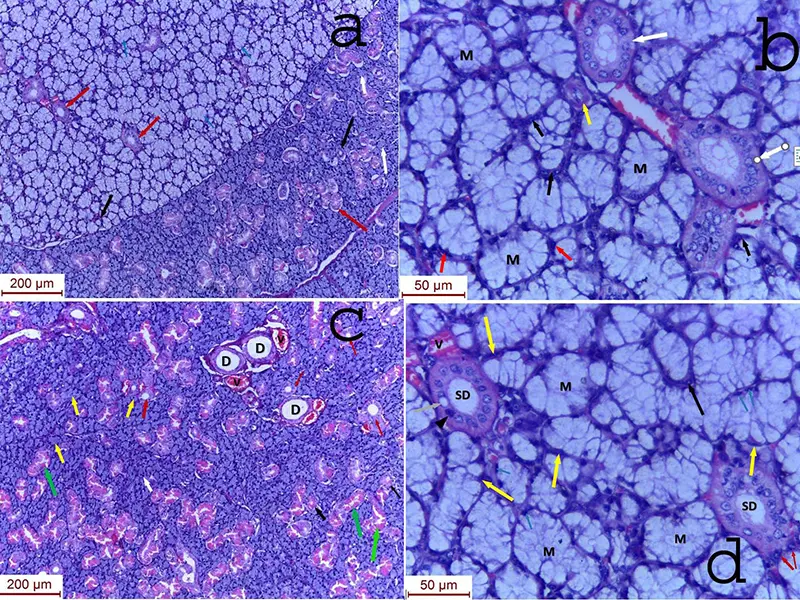
In the nasal mucosa, ZIO positive LCs were seen in the surface epithelium, lamina propria and among the glands (Fig. 2). All the types of LCs as described by Figueroa and Caorsi (Figueroa and Caorsi, 1980), were seen in both the epithelium and lamina propria (Fig. 3). They were classified as follows: Type 1: Single process, unbranched; Type 2: One process divided into branches; Type 3: Two processes; Type 4: Three or more processes; Type 5: Three or more processes with arborization.
In addition, non-dendritic LCs were also identified in the nasal mucosa. In some cases of polyp due to allergic fungal rhinosinusitis, ZIO positive LCs were aggregated just beneath the basement membrane (Fig. 4). A case of rhinosinusitis with polyposis showed subepithelial stroma with oedema, congestion, focal fibrosis and several thick- or thin-walled blood vessels. It also had lymphatic aggregation in the lamina propria, in which non-dendritic types of ZIO positive LCs were seen in the periphery of the follicle (Fig. 5).
The ZIO positive LCs were seen interspersed among seromucous glands in the polypoid group (Fig. 2). They were observed along the walls of the glands and also within it. In a cystically dilated gland they were embedded along its wall (Fig. 6).
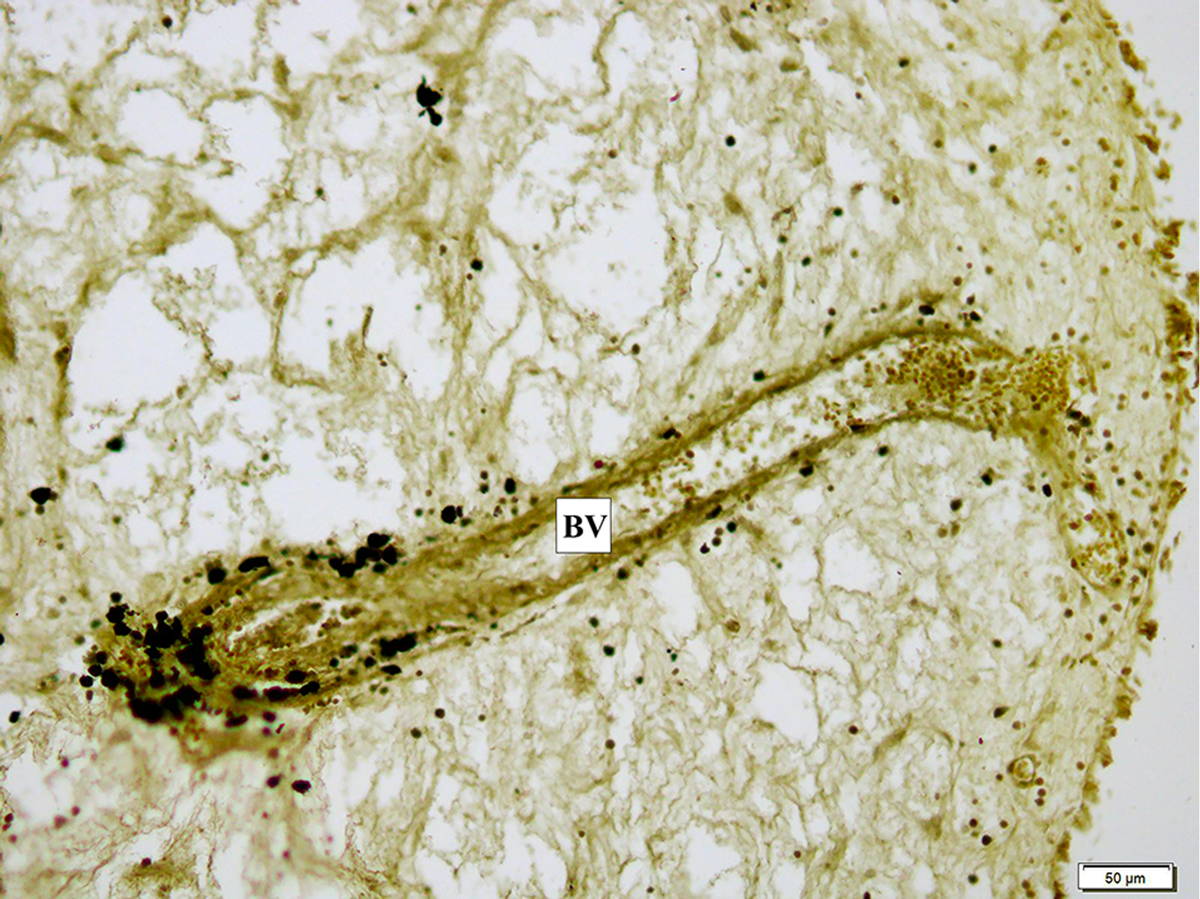
The ZIO positive LCs were seen clustered in and around these blood vessels. Many of them were non-dendritic in nature, large and polygonal in shape (Fig. 7). The number of ZIO positive LCs in the polypoid group ranged from 0 to 97 with a mean of 24.38 ± 29.51 per mm2 (Table 1). On comparing the distribution of ZIO positive LCs between the two groups, a significantly higher number of ZIO positive LCs was noted in group II patients (p=0.001). This shows that polyposis due to whatever aetiology is associated with a higher density of ZIO positive LCs (Table 1).
DISCUSSION
The dendritic cells present in respiratory mucosa are essential for stimulating naive T cells in a primary immune response against inhaled allergens and for the development of allergic sensitization. A subset of dendritic cells called LCs, are present in different mucosal linings including the nasal mucosa (Fokkens et al., 1989a, b). Though they play a crucial role as critical regulators of many inflammatory diseases, only a limited understanding exists regarding the role of LCs in sinonasal polyposis. Of the many types of LCs, ZIO positive LCs are a distinct subtype demonstrated in other tissues like cervix, Fallopian tube and appendix and are known for their immunoregulatory role. In this study, we examined the density of ZIO positive LCs in the nasal mucosa to determine the difference between non-polypoid and polypoid groups.
LCs in the nasal mucosa
Fokkens et al. (1989a, b) had demonstrated the CD1a positive LCs in the middle and basal layers of the pseudostratified ciliated columnar epithelium of normal nasal mucosa. In the present study, the ZIO positive cells were present in the pseudostratified ciliated columnar epithelium of the polypoid group, but they were nearly absent in the non-polypoid group. The authors have previously demonstrated that ZIO positive LCs cells are different from CD1a positive LCs in their distribution, and suggested that they are different subsets of LCs and may have different immunological roles (Rabi et al., 2014a). Additionally, all the five types of typical dendritic type of ZIO positive LCs were noted in the nasal mucosa, predominantly in the lamina propria. Godthelp et al. (1996) and Fokkens et al. (1989a, b) reported a significant increase of LCs and HLA-DR positive cells in the lamina propria of patients with allergic rhinitis.
The ZIO positive LCs have not been demonstrated in the pseudostratified ciliated columnar epithelium so far, but have been demonstrated in other epithelia such as the stratified squamous epithelium of lip, tongue, oesophagus, cervix and eyelid (Indrasingh et al., 2001; Rabi et al., 2014b, c), and the simple columnar epithelium of the small intestine and appendix (Koshy et al., 2020; Rabi et al., 2019). In this study, these cells were nearly absent in pseudostratified ciliated columnar epithelium of group I patients, but were seen in the hyperplastic pseudostratified ciliated columnar epithelium. They were also noted in the pseudostratified ciliated columnar epithelium in patients with polyps.
LCs in lymphatic aggregation of nasal polyps
Nasal/ nasopharynx associated lymphoid tissue (NALT) is a component of the immune system of the nasal mucosa and is part of the mucosa-associated lymphoid tissue (MALT) present in all mammals, which protects the body from airborne viruses and other infectious agents. It serves as a target site for local defence strategies and vaccine induction sites (Debertin et al., 2003). To begin an immune response, the maturing LCs primarily migrate to the lymphoid tissue where antigens are presented to the lymphocytes (Yao et al., 2002). The antigenic dose, the distribution of antigen that targets distinct types of dendritic cells present in the NALT, may contribute to the difference in the immunological outcome to an inhaled antigen (Iwasaki, 2007).
This study noted that where there is an infiltration of inflammatory cells like mast cells neutrophils and lymphocytes associated with subepithelial stromal oedema and focal fibrosis, non-dendritic ZIO positive LCs were noted within the lymphatic aggregation in cases of nasal polyps. The ZIO positive non-dendritic follicular dendritic cells were observed in the germinal centre and dendritic follicular cells in the mantle zone of the normal appendix. The authors had suggested that the difference in morphology could be due to functional difference (Rabi et al., 2019). Linder et al. (1993) had also reported the presence of numerous scattered T lymphocytes and HLA-DR positive dendritic-like cells in the subepithelial areas of both nasal polyp and normal nasal mucosa. The proximity of LCs to the T lymphocytes indicates that cellular-immune responses are initiated by direct interaction of naive T cells and LCs in nasal mucosa (Iwasaki, 2007). Presence of NALT in nasal mucosa has to be extensively researched in humans.
LCs in nasal mucosal glands
The present study showed ZIO positive LCs interspersed among the seromucous glands of lamina propria, more in the polypoid group. They were noted within the glands and on their walls in both groups. The ZIO positive LCs were studded within the cystically dilated glands filled with secretory material and on their walls in the polypoid group. Similarly, Linder et al. demonstrated an aggregation of T lymphocytes and HLA-DR-positive cells adjacent to the deeper seromucous glands (Linder et al., 1993). This may suggest antigen presentation to the lymphocytes around the glands.
Vascular LCs in nasal mucosa
In the present study, ZIO positive LCs were seen surrounding some blood vessels and capillaries. Vascular dendritic cells in the arterial intima were identified with anti-CD1a and S-100 (Bobryshev and Lord, 1995a, b) way back in 1995. They were located at regular intervals along the subendothelial layer, and were often in close contact with endothelial cells (Bobryshev and Lord, 1995b).
Rabi et al. (2014a) reported ZIO positive LCs surrounding the intraepithelial capillaries in the human cervix. These findings advocate the possibility of antigen presentation occurring even around the blood vessels.
Morphology of LCs in nasal mucosa
The ZIO positive LCs in this study were of both dendritic and non-dendritic nature in both groups. Mathew et al. had demonstrated the presence of non-dendritic and dendritic subtypes of CD1a positive LCs in the buccal mucosa (Mathew et al., 2019). The non- dendritic types of ZIO positive LCs were previously described by Rabi et al. (2014b) in the mucosa of the human exocervix. Shortman and Naik (2007) classified the dendritic cells into conventional dendritic cells and precursors of dendritic cells in his study. They described that the precursor dendritic cells, although without a dendritic form, could have an inherent capacity to develop into conventional dendritic cells at the onset of an inflammatory or microbial stimulus.
All the types of typical dendritic cells as described by Figueroa and Caorsi (1980) were identified in this study. The typical dendritic ZIO positive LCs were plenty in the nasal mucosa of the polypoid group. This is the first report of the ZIO positive LCs and their typical dendritic types in the nasal mucosa to the best of our knowledge.
In the present study, large polygonal non-dendritic types of ZIO positive LCs were noted in the epithelium, lamina propria including lymphatic aggregation and in and around blood vessels. These cells could be considered as a different subset of LCs or accessory antigen presenting cells. Extensive research in the field of non- dendritic LCs and their functions remains an unsolved question augmenting the need for further in vivo studies. A thorough understanding of specific molecular and cellular properties of NALT is essential for the development of an effective intranasally administered vaccine. NALT-targeted immunization efficiently induces antigen-specific immune responses in both mucosal and systemic immune compartments. Presence of high density of LCs in the nasal mucosa is essential for efficient antigen uptake, which is a necessary criterion for nasal immunotherapy.
CONCLUSION
The current study shows the distribution and characteristics of ZIO positive LCs in patients with polypoid disease of the nose and sinuses, and highlights the difference in distribution of these LCs in normal mucosa and patients with sinonasal polyposis. The demonstration of ZIO positive LCs confirms the presence of immunoregulatory mechanisms that come into play in inflammatory sinus disease.
ACKNOWLEDGEMENTS
This work was supported by the Fluid Research Committee, Christian Medical College, Vellore. The authors would also like to acknowledge the contributions of Dr Meera Thomas, Professor, Department of Pathology, Christian Medical College, Vellore for providing assistance in data interpretation.
Related articles
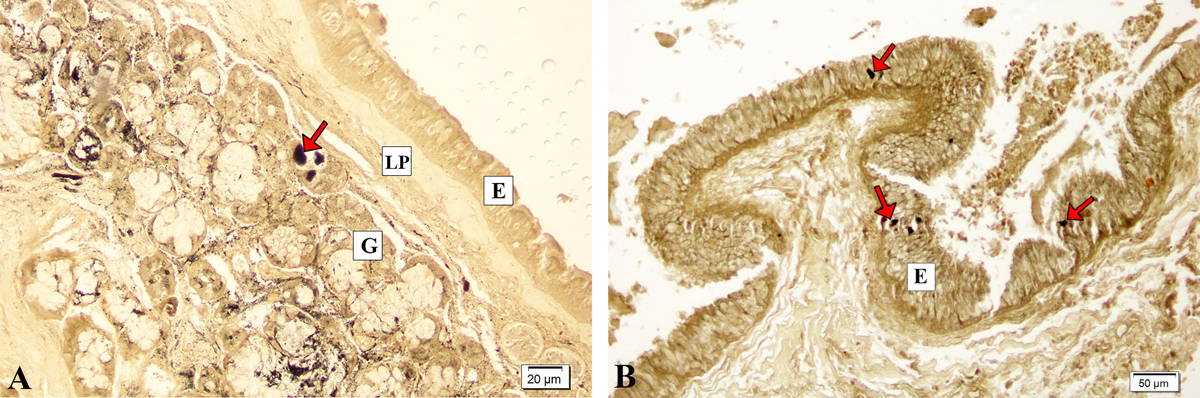 Fig. 1.- A. Arrow indicating ZIO positive LCs in the mucosal glands (G) of normal nasal mucosa. E- pseudostratified ciliated columnar epithelium; LP- lamina propria. B. Hyperplastic pseudostratified ciliated columnar epithelium of non-polypoid nasal mucosa. The arrows indicate ZIO positive LCs in the epithelium (E).
Fig. 1.- A. Arrow indicating ZIO positive LCs in the mucosal glands (G) of normal nasal mucosa. E- pseudostratified ciliated columnar epithelium; LP- lamina propria. B. Hyperplastic pseudostratified ciliated columnar epithelium of non-polypoid nasal mucosa. The arrows indicate ZIO positive LCs in the epithelium (E). Fig. 2.- Nasal mucosa of a nasal polyp. Arrows indicating ZIO positive LCs in the surface epithelium (E), lamina propria (LP) and among glands (G).
Fig. 2.- Nasal mucosa of a nasal polyp. Arrows indicating ZIO positive LCs in the surface epithelium (E), lamina propria (LP) and among glands (G). Fig. 3.- A. Type 1(single process, unbranched) and Type 4 (with three or more processes) LCs are indicated by arrows. E- Epithelium. B. Type 2 LC (one process which divides into branches) indicated by an arrow Type 3 LC (Two processes). E- Epithelium. C. Type 5 LC (with three or more processes with arborization) indicated by an arrow. E- Epithelium.
Fig. 3.- A. Type 1(single process, unbranched) and Type 4 (with three or more processes) LCs are indicated by arrows. E- Epithelium. B. Type 2 LC (one process which divides into branches) indicated by an arrow Type 3 LC (Two processes). E- Epithelium. C. Type 5 LC (with three or more processes with arborization) indicated by an arrow. E- Epithelium.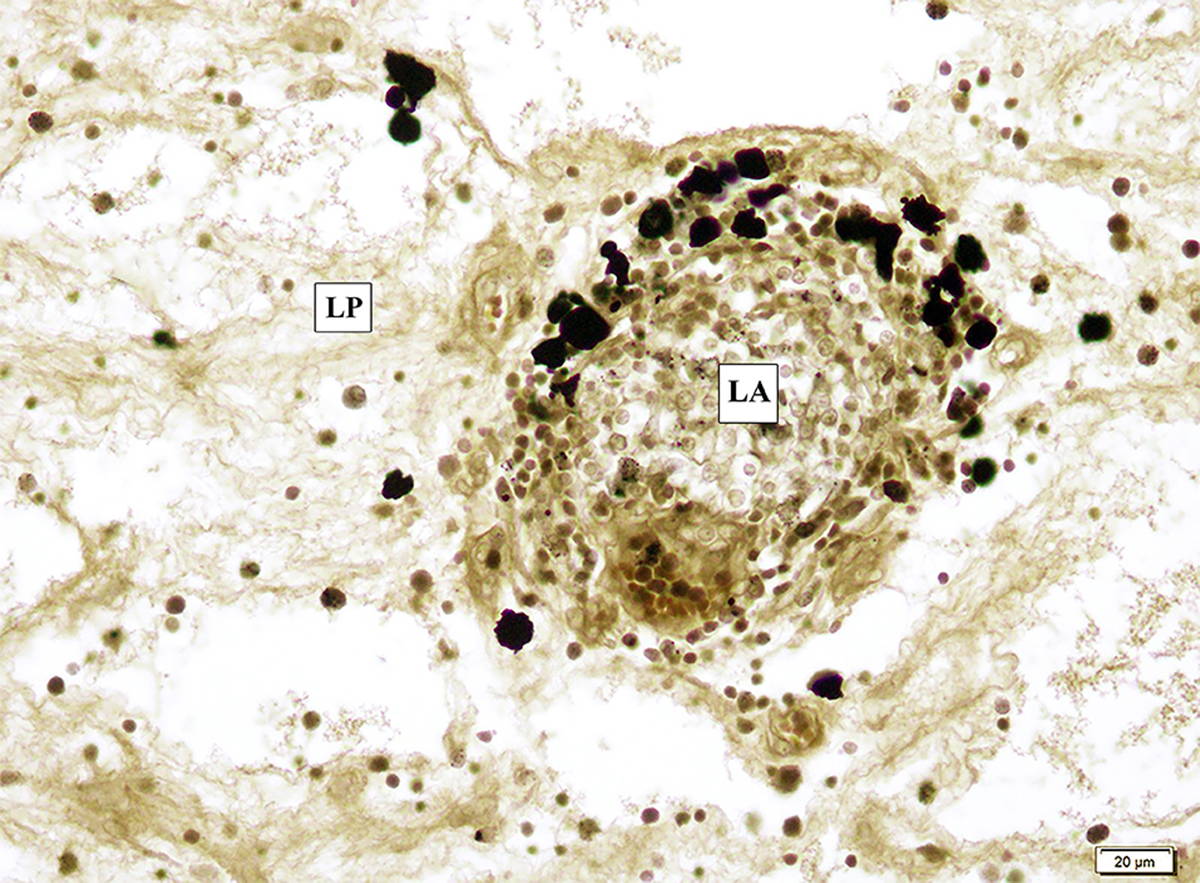 Fig. 5.- Non dendritic ZIO positive LCs among the lymphatic aggregates (LA) in the lamina propria (LP) in a case of nasal polyp.
Fig. 5.- Non dendritic ZIO positive LCs among the lymphatic aggregates (LA) in the lamina propria (LP) in a case of nasal polyp.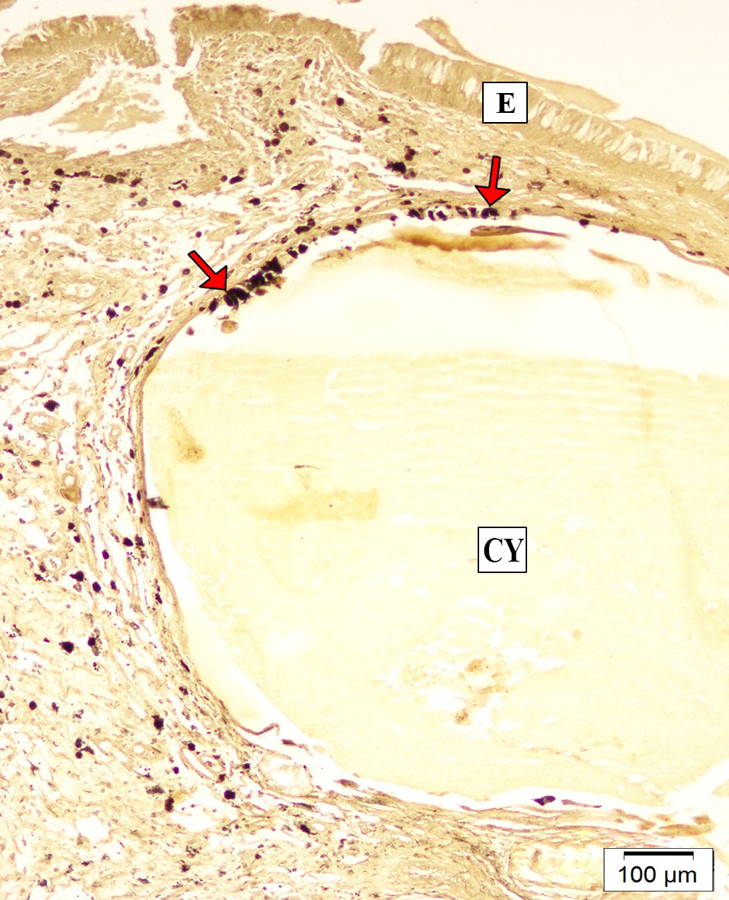 Fig. 6.- The arrows indicate ZIO positive LCs in the walls of a cystically dilated gland (CY) filled with secretory material. E- Epithelium.
Fig. 6.- The arrows indicate ZIO positive LCs in the walls of a cystically dilated gland (CY) filled with secretory material. E- Epithelium.ADEMA GJ (2009) Dendritic cells from bench to bedside and back. Immunol Lett, 122(2): 128-130.
BOBRYSHEV YV, LORD RS (1995a) S-100 positive cells in human arterial intima and in atherosclerotic lesions. Cardiovas Res, 29(5): 689-696.
BOBRYSHEV YV, LORD RS (1995b) Ultrastructural recognition of cells with dendritic cell morphology in human aortic intima. Contacting interactions of vascular dendritic cells in athero-resistant and athero-prone areas of normal aorta. Arch Histol Cytol, 58(3): 307-322.
DEBERTIN AS, TSCHERNIG T, TÖNJES H, KLEEMANN WJ, TRÖGER HD, PABST R (2003) Nasal-associated lymphoid tissue (NALT): frequency and localization in young children. Clin Exp Immunol, 134 (3): 503-507.
FIGUEROA CD, CAORSI I (1980) Ultrastructural and morphometric study of the Langerhans cell in the normal human exocervix. J Anat, 131(Pt 4): 669-682.
FOKKENS WJ, VROOM T, RIJNTJES E, MULDER P (1989a) Fluctuation of the number of CD-1(T6)-positive dendritic cells, presumably Langerhans cells, in the nasal mucosa of patients with an isolated grass-pollen allergy before, during, and after the grass-pollen season. J Allergy Clin Immunol, 84(1): 39-43.
FOKKENS WJ, VROOM THM, RIJNTJES E, MULDER PGH (1989b) CD-1 (T6), HLA-DR-expressing cells, presumably Langerhans cells, in nasal mucosa. Allergy, 44(3): 167-172.
GODTHELP T, FOKKENS WJ, KLEINJAN A, HOLM AF, MULDER PGH, PRENS EP, RIJNTES E (1996) Antigen presenting cells in the nasal mucosa of patients with allergic rhinitis during allergen provocation. Clin Exp Allergy, 26(6): 677-688.
INDRASINGH I, ABRAHAM S, VETTIVEL S (2001) Zinc iodide osmium positive cells and dendritic cells in stratified squamous epithelium of lip, tongue, and oesophagus of bonnet monkey (Macaca Radiata). J Anat Soc India, 50: 34-36.
IWASAKI A (2007) Mucosal dendritic cells. Ann Rev Immunol, 25(1): 381-418.
JAITLEY, SHWETA, SARASWATHI TR (2012) Pathophysiology of Langerhans cells. J Oral Maxillofac Pathol, 16(2): 239-244.
KOSHY S, INDRASINGH I, VETTIVEL S (2003) Dendritic cells in the human ileum: a light microscopic zinc iodide-osmium study. Eur J Anat, 7(3): 127-130.
LINDER A, KARLSSON-PARRA A, HIRVELÄ C, JONSSON L, KÖLING A, SJÖBERG O (1993) Immunocompetent cells in human nasal polyps and normal mucosa. Rhinology, 31(3): 125-129.
MATHEW JK, RAJADOSS MKP, PRANAY G, SUGANTHY R, TAMIL N (2019) Non-dendritic Langerhans cells: a new entity in normal and malignant buccal mucosa. Eur J Anat, 23(5): 383-388.
ÖNERCI, METIN (2010) Nasal Polyposis. Springer-Verlag, Berlin, Heidelberg.
O’RAHILLY R, MÜLLER F, CARPENTER S, SWENSON R (1967) The nose and paranasal sinuses. 2nd ed. Basic Human Anatomy, A Regional Study of Human Structure. WB Saunders Co., Philadelphia, pp 924-932.
PEZATO R, CLAUDINA AP, GABRIELE H, NATALIE DR, KOEN VC, GEERT DV, CLAUS B, LARA D (2014) The expression of dendritic cell subsets in severe chronic rhinosinusitis with nasal polyps is altered. Immunobiology, 219(9): 729-736.
RABI S, TRIPTI MJ, LIONEL J, INDRASINGH I (2014a) Different subsets of Langerhans cells in human uterine tubes and uterus. J Obstet Gynaecol Res, 40: 1833-1839.
RABI S, TRIPTI MJ, INDRASINGH I (2014b) Demonstration of CD 1a-positive and zinc iodide-osmium-positive Langerhans cells in the human eyelid. Clin Exp Ophthalmol, 42(8): 802-804.
RABI S, LIONEL J, INDRASINGH I (2014c) Morphological study of dendritic cells in human cervix by zinc iodide osmium method. J Clin Diagn Res, 8(6): AC01-04.
RABI S, INDRASINGH I, KOSHY S, SUGANTHY J (2019) Distribution of zinc iodide-osmium positive dendritic cells in the human appendix. Eur J Anat, 10(1): 15-20.
SCHROEDER JT, BIENEMAN AP, XIAO H, CHICHESTER KL, VASAGAR K, SAINI S, MARK CL (2005) TLR9- and FcεRI-mediated responses oppose one another in plasmacytoid dendritic cells by down-regulating receptor expression. J Immunol, 175(9): 5724-5731.
SHORTMAN K, SHALIN HN (2007) Steady-state and inflammatory dendritic-cell development. Nat Rev Immunol, 7(1): 19-30.
YAO V, CAMERON P, JOHN CH (2002) Dendritic cells. ANZ J Surg, 72(7): 501-506.