The number and organization of sarcomeres within a muscle (referred to as muscle architecture) can be used to predict functional capability. The six deep external rotator muscles of the hip (piriformis (PI), quadratus femoris (QF), obturator internus (OI), obturator externus (OE), superior gemellus (SG), and inferior gemellus (IG)) play a role in both hip stabilization and rotation and are damaged and relocated during total hip arthroplasty surgery. Understanding the architectural details of these muscles could lead to improved clinical understanding of their function. Therefore, muscles were excised from 12 embalmed cadavers (6 male, 6 female aged 56-88 years) to measure muscle mass, fascicle length, and sarcomere length. These variables were used to calculate the architectural parameters physiological cross-sectional area (PCSA) and normalized fascicle length (LFn). Results demonstrated that in the measured neutral cadaveric posture all six muscles had mean sarcomere lengths (2.40 to 2.57 μm) that placed them on the ascending limb of the force-length relationship where they could theoretically generate more than 90 percent of their maximum capable force. The OI had the largest PCSA, and thus largest force-generating capability, while the PI had the longest normalized fascicle length, and thus largest excursion and shortening velocity capabilities. SG and IG were the smallest in both regards. These data provide valuable insight into the force-generating capability of the hip deep external rotator muscles and can be used as inputs to biomechanical models to predict function in healthy movement or within a variety of clinical conditions.
Deep external rotator muscles of the hip: an anatomical and architectural study
Ian A. Scagnetti, Lorraine C. Jadeski, Stephen H.M. Brown
Department of Human Health and Nutritional Sciences, University of Guelph, Guelph, ON, Canada
SUMMARY
Sign up or Login
INTRODUCTION
External rotation of the femur and stabilization of the hip joint are performed in part by the group of six muscles defined anatomically as the deep external rotators of the hip (Dostal, 1987; Agur and Dalley, 2016). These muscles are: piriformis (PI), quadratus femoris (QF), obturator internus (OI), obturator externus (OE), superior gemellus (SG), and inferior gemellus (IG) (Fig. 1). These six muscles extend between their attachments at various points on the ischium and pubis and the greater trochanter, trochanteric fossa and quadrate tubercle of the femur. The bony attachments make it possible for the muscles to cross the hip joint from several different angles, thereby facilitating the generation of external rotation throughout the hip joint’s range of motion. These muscles also work to stabilize the head of the femur within the acetabulum (Agur and Dalley, 2016). This important group of muscles is damaged during total hip arthroplasty surgery (THA) in varying levels of severity depending on the surgical approach and technique employed (Bottner and Pellici, 2006; Meneghini et al., 2006), and as a result, long-term weakness and instability of the hip joint is often observed in THA patients’ post-surgery (Winther et al., 2016; Kawasaki et al., 2017). In Canada alone, approximately 55,000 THA surgeries are done per year, and 4,500 revision surgeries performed per year due to failure in stability of the hip joint (Canadian Institute for Health Information, 2017). Unfortunately, the architecture of the deep hip external rotator muscle group has not been well documented.
Muscle architecture describes the internal organization of contractile elements called sarcomeres. The number and organization of sarcomeres within a muscle can be used to predict a muscle’s functional capacity regarding the excursion and velocity potential of the muscle fibres, as well as the ability of the muscle to produce force. The best predictor of a muscle’s excursion and velocity potential is normalized fascicle length (LFn), which represents the number of sarcomeres arranged in-series along the length of the muscle. The best predictor of a muscle’s ability to produce force is its physiological cross-sectional area (PCSA), which represents the number of sarcomeres arranged in-parallel within the muscle. Mean sarcomere length also plays an important role in determining a muscle’s ability to produce force, as described by the well-known sarcomere force-length relationship (Gordon et al., 1966; Walker and Schrodt, 1974). These important architectural variables have not been well defined for the deep external rotator muscles of the hip.
Therefore, the current study was designed to measure the mass, sarcomere length and fascicle length of the six external rotator muscles of the hip from cadaveric donors, and use those measurements to calculate the architectural parameters normalized fascicle length and physiological cross-sectional area.
MATERIALS AND METHODS
All dissection and photography was performed in the University of Guelph Human Anatomy Laboratory. Permission is given by the Chief Coroner of Ontario to use cadaveric specimens donated to the University of Guelph for research purposes. Permission is also given by whole body donors and their families via the Anatomy Act (Province of Ontario) to utilize tissue for scientific research at the University of Guelph.
The cadavers in this study were embalmed by arterial perfusion using a 2.5% formaldehyde solution (60% ethanol, 30% propylene glycol, 5% phenol, 2.5% formaldehyde, 2.5% Dettol disinfectant), which results in approximate muscle density of 1.112 gm/cm3 (Ward and Lieber, 2005). The current study followed the protocol for muscle dissection and removal described by Sacks and Roy (1982) in their study on feline hind-limb muscles and previously used for human muscles by multiple authors (e.g. Ward et al., 2009). The six muscles were harvested from one side of the body of each of 12 formaldehyde-fixed human cadavers [6 male and 6 female aged 56 – 88 years (mean = 79.3 years)] by carefully dissecting the muscles away from their origins and insertions using hand tools. Specimens were excluded if the medical history of the body donor reported any hip-related injury, surgery, chronic condition or wheelchair confinement. Specimens were also excluded if damage to the muscle or hip joint was observed during dissection. After the muscles were removed from their bony attachments, they were dissected to remove as much superficial fat and connective tissue as possible. Each muscle was then weighed to an accuracy of ±0.01 g using a digital scale. Next, using digital calipers (resolution of 0.01 mm), fascicle length was measured at three different locations (one central and two lateral) for each muscle (Fig. 2). The fascicle measurements were taken in these proximal, middle and distal areas of the muscle belly to represent the average fascicle length. To measure sarcomere length, three small biopsies were taken from each of the three fascicle regions described above (example of proximal, middle and distal locations along the fascicle marked with asterisks on the obturator externus muscle shown in Figure 2) and subjected to laser diffraction (Baskin and Lieber, 1983). Measured fascicle lengths and sarcomere lengths were averaged to provide a representative value for each muscle.
The mean fascicle length was normalized to the optimal sarcomere length for human muscle (2.7µm) using the equation below (Ward et al., 2009):
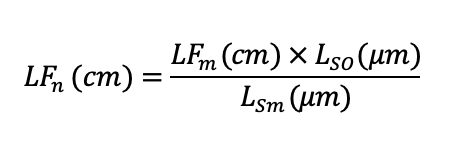
Where LFn represents normalized fascicle length, LFm represents measured fascicle length, Lso represents optimal sarcomere length for human muscle (2.7 μm; Walker and Schrodt, 1974) and Lsm represents measured sarcomere length.
Each muscle’s PCSA was then determined using the following equation (Ward and Lieber, 2005):
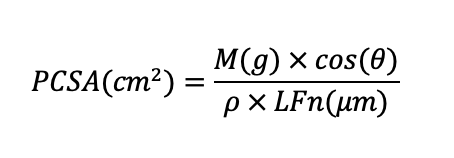
Where M is the muscle mass in grams (g), θ is the angle of pennation of the muscle fibres (negligible for the muscles studied here), LFn is the average normalized fascicle length of the muscle, and ρ is muscle density (gm/cm3).
Mean sarcomere lengths were also represented relative to the established sarcomere force-length relationship for human muscle. Based on the cross-bridge theory of muscle contraction, the shape of this relationship is governed by the overlap between actin and myosin proteins (Gordon et al., 1966); this overlap is dependent upon the length of these proteins. As these lengths are relatively consistent across muscles within a species, a defined force-length relationship can be presented (Walker and Schrodt, 1974; Burkholder and Lieber, 2001).
Using a two-way mixed-model analysis of variance (ANOVA) test, independent variables (muscle, sex) were compared within each dependent variable (architectural parameters: sarcomere length, PCSA, LFn) for all cadavers (n=12) with muscle as a repeated factor for each cadaver. If significant differences were found (p < 0.05), Tukey post-hoc tests were conducted where appropriate.
RESULTS
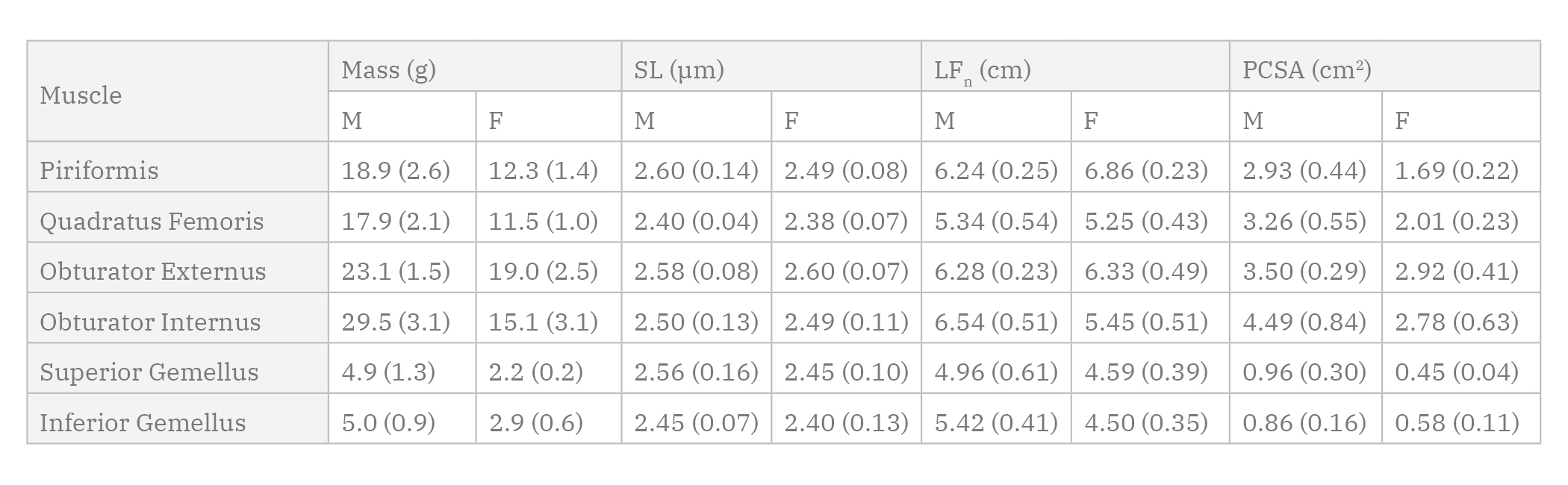
Mean architectural values, separated for males and females, of the six deep hip external rotator muscles fixed in neutral cadaveric posture are found in Table 1.
A significant effect of sex on PCSA was found, with muscles from male donors demonstrating greater mean PCSA than muscles from female donors (p < 0.0001), indicating a greater capacity for force production in male muscles (Fig. 3). There were also significant differences in PCSA amongst muscles of all donors (p < 0.0001) (OI > PI, SG, and IG; OE, PI, and QF > SG, IG). There was no statistically significant interaction effect for PCSA (p = 0.23).
There was no significant difference in LFn between male and female muscles (p = 0.14) (Fig. 4). There was a significant main effect of muscle (p = 0.0002) for LFn amongst muscles (PI > QF, SG, and IG; OE > SG and IG). There was no statistically significant interaction effect for LFn (p = 0.27).
There were no significant differences in sarcomere length amongst muscles (p = 0.26), nor between sexes (p = 0.29), nor a significant interaction (p = 0.98) between muscle and sex. Mean sarcomere lengths for each muscle were between 2.40µm and 2.57µm, indicating that they acted between 90.7% and 96.1% of their maximal ability to produce force, according to the well-established force-length curve (Gordon et al., 1966; Walker and Schrodt, 1974). Mean sarcomere lengths of each muscle from all donors are shown with their relative positions on the force-length curve in Fig. 5.
Mean PCSA and LFn for each muscle from all donors, separated by sex, are plotted in Fig. 6.
DISCUSSION
This is the first study to examine and provide a high-resolution data set for the muscle architecture of the deep external rotator muscles of the hip. Interpretation of muscle functional capability, in terms of force generating capability and excursion potential, can be interpreted based on the architectural parameters PCSA and normalized fascicle length, respectively, and Figure 6 is provided to facilitate this comparison for both male and female muscles. Specifically, this enables visualization of which muscles are best suited to generating high forces and which muscles are best suited to generating force over a wide range of lengths and at high velocities. Further, these data can provide input for the development of biomechanical models of the pelvis and lower limb to study a variety of topics ranging from gait related disorders, childbirth and related muscle weakness, to surgical techniques including posterior approach total hip arthroplasty that involves the cutting and re-attachment of the PI, OI, SG, and IG.
Expected differences were observed between male and female muscles regarding PCSA. PCSA was significantly greater for males (p < 0.0001), indicating that male deep external hip rotator muscles are able to produce more force than female muscles. This result was expected, because PCSA is largely dictated by the mass of the muscle, which is generally correlated to the mass of the whole body (Janssen et al., 2000). There were also significant differences in PCSA amongst muscles of all donors (p < 0.0001) (OI > PI, SG, and IG; OE, PI, and QF > SG, IG). Thus, it can be concluded that the OI has the greatest force generating potential while SG and IG have the lowest.
Significant differences (p = 0.0002) were observed between the LFn of the deep hip external rotator muscles (PI > QF, SG, and IG; OE > SG and IG), indicating that PI has the greatest excursion and contraction velocity potentials and SG and IG have the least. No statistically significant differences were found for normalized fascicle length between males and females, which was of itself interesting. Males on average have greater body height, and therefore body segments and associated muscles would be expected to be longer (Statistics Canada, 2008). However, this was not the case for the hip external rotator group (Figure 4). A potential explanation for this lack of sex-based difference in normalized fascicle length relates to the differences in the anatomy of male and female pelvises. The difference in male and female pelvic bone shape observed during dissection for this study is well documented (DeSilva and Rosenberg, 2017). Namely, the male pelvis is generally taller and narrower than the female pelvis, with anterior angling of the sacrum. Conversely, the female pelvis is generally shorter and wider than the male pelvis, and has a more posterior angle of the sacrum to accommodate childbirth. The increased width of the female pelvis results in a broader and more lateral position of the hip joints. These differences in pelvis shape appear to offset expected differences in overall body height and ultimately result in normalized muscle fascicle lengths (LFn) of the deep hip external rotator muscles that are not different between males and females.
The mean sarcomere length of a muscle in neutral cadaveric posture can be used to infer the muscles’ ability to produce contractile force (Gordon et al., 1966). Each of the six muscles had mean sarcomere lengths, measured in the neutral cadaveric posture, between 2.40-2.57µm, indicating that in this position they were all capable of generating greater than 90 % of their maximum isometric force based on their location on the ascending limb of the force-length curve. This reveals that as the muscles are initially lengthened during movement or potential hip joint instability, they will become more effective at producing force until they surpass approximately 2.8µm, at which point force production capability would drop off. Interestingly, Suh et al (2004) demonstrated that reattachment of the external rotator muscles to the greater trochanter via a pair of transosseous sutures significantly lowered the chance of hip dislocation post-THA surgery. It is possible that the sarcomere length data presented in the current study could be used to further improve function of these muscles after reinsertion; specifically, intraoperative sarcomere length measurements (Lieber and Friden, 2001) have the potential to increase the likelihood that muscles are reattached at their natural in vivo lengths. Exploring this might further reduce the muscle weakness and instability that can be observed in patients after THA surgery. The results of this study are limited by the age-range of the individuals from which the specimens were collected (mean 79.3 years; range 56-88 years). Therefore, the data reported here are representative of an older demographic. If and how these would change in a younger group is unclear, but one previous study of the OI has demonstrated that PCSA was significantly lower in a group older than 60 years of age compared with younger groups, with no changes in LFn or sarcomere length with age (Cook et al., 2017). Based on this, it is reasonable to expect a younger population to have larger PCSAs of all of the hip external rotator muscles than those reported here, but there is nothing to suggest that mean sarcomere lengths or LFns would be different between younger and older populations.
In summary, this study has yielded a high-quality muscle architecture data-set for the deep hip external rotator muscle group. Sarcomere lengths measured in the neutral cadaveric posture were between 2.40µm and 2.57µm for all six muscles, indicating that they act in this position on the ascending limb of the force-length relationship where they can generated greater than 90% of their maximal isometric force. OI had the greatest force-generating capability and PI the greatest length excursion and velocity potentials, while SG and IG were the smallest in both regards. The data reported here are required parameters that can be used to add these muscles to existing software models of the hip joint (e.g. OpenSim; Delp and Loan, 1995) which can be used to improve the understanding of the functional importance of these muscles and their potential role in preventing or treatment of hip disorders, including post-THA surgery (e.g. Myers et al., 2019).
Related articles
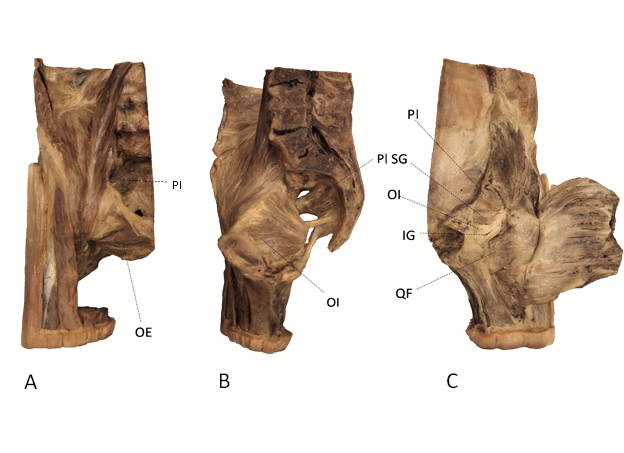 Fig. 1.- (A) Anterior view of right-side bisected pelvis, origin of piriformis (PI) and origin of obturator externus (OE) shown. (B) Mid-sagittal view of right-side bisected pelvis, origin of obturator internus (OI), and origin of piriformis (PI) shown. (C) Posterior view of right-side bisected pelvis, insertions of piriformis (PI), superior gemellus (SG), obturator internus (OI), inferior gemellus (IG) and quadratus femoris (QF) shown.
Fig. 1.- (A) Anterior view of right-side bisected pelvis, origin of piriformis (PI) and origin of obturator externus (OE) shown. (B) Mid-sagittal view of right-side bisected pelvis, origin of obturator internus (OI), and origin of piriformis (PI) shown. (C) Posterior view of right-side bisected pelvis, insertions of piriformis (PI), superior gemellus (SG), obturator internus (OI), inferior gemellus (IG) and quadratus femoris (QF) shown. Fig. 2.- Excised obturator externus muscle (left side, anterior view) with fat, connective tissue and tendon removed. Yellow dotted lines indicate measurement locations for fascicle length, and asterisks indicate locations biopsied for sarcomere length measurement. Similar fascicle locations and biopsy locations were chosen for measurement from each muscle in the current study..
Fig. 2.- Excised obturator externus muscle (left side, anterior view) with fat, connective tissue and tendon removed. Yellow dotted lines indicate measurement locations for fascicle length, and asterisks indicate locations biopsied for sarcomere length measurement. Similar fascicle locations and biopsy locations were chosen for measurement from each muscle in the current study..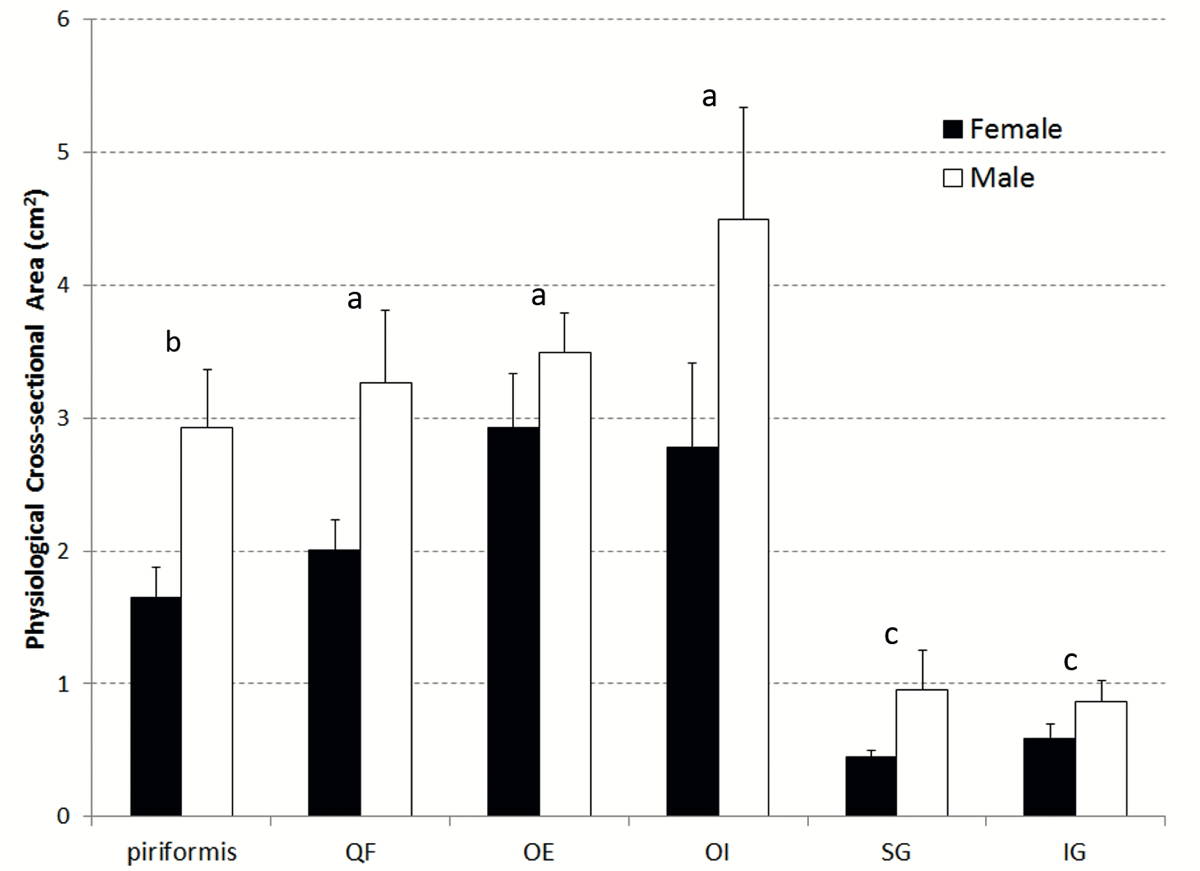 Fig. 3.- Mean (+ Standard Error of the Mean (SEM)) PCSA for both male and female muscles. Male and female were significantly different for all muscles. The same letters above the bars indicate muscles were not significantly different from one another.
Fig. 3.- Mean (+ Standard Error of the Mean (SEM)) PCSA for both male and female muscles. Male and female were significantly different for all muscles. The same letters above the bars indicate muscles were not significantly different from one another.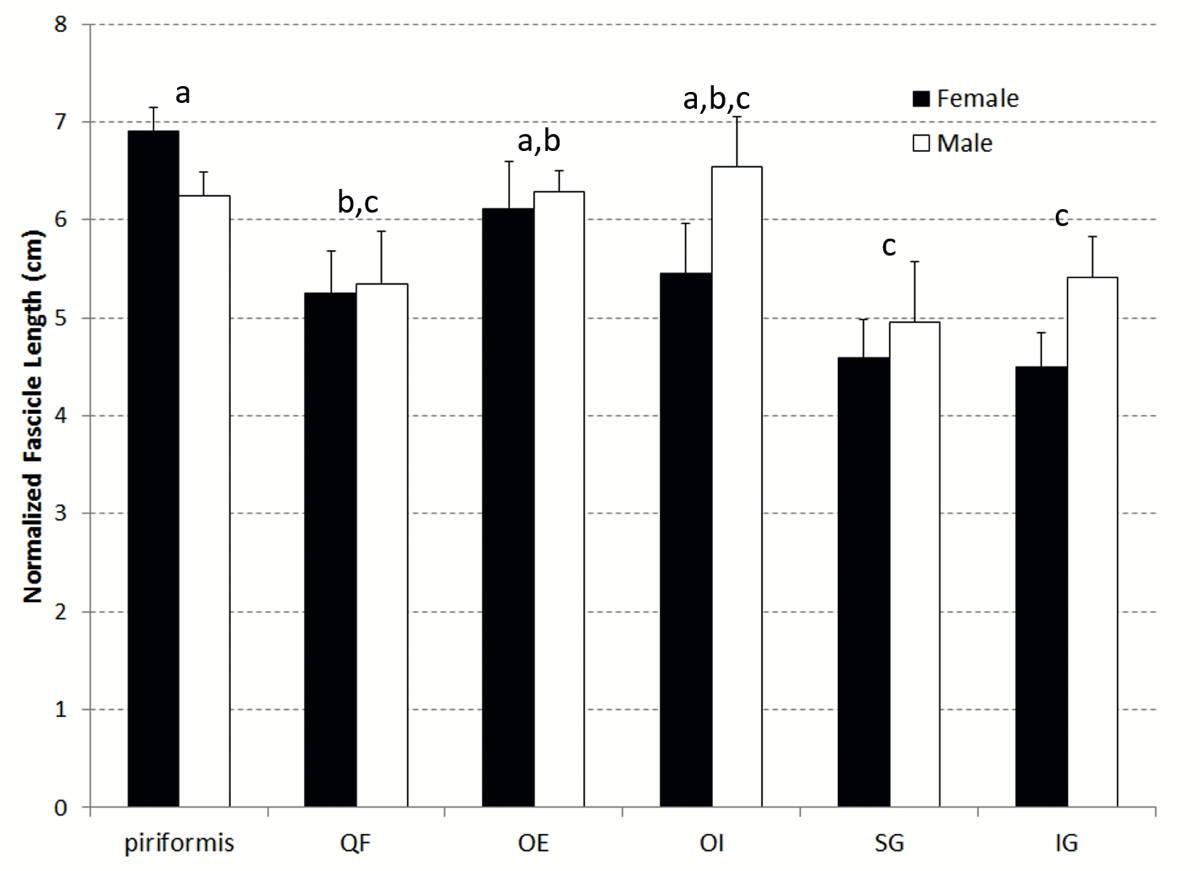 Fig. 4.- Mean (+ SEM) normalized fascicle length for both male and female muscles. The same letters above the bars indicate muscles were not significantly different from one another.
Fig. 4.- Mean (+ SEM) normalized fascicle length for both male and female muscles. The same letters above the bars indicate muscles were not significantly different from one another. Fig. 5.- Mean sarcomere length for each muscle represented on the force-length curve as a percentage of maximal force production capable by the sarcomere in neutral cadaveric posture.
Fig. 5.- Mean sarcomere length for each muscle represented on the force-length curve as a percentage of maximal force production capable by the sarcomere in neutral cadaveric posture. ABDELMOHSEN AM (2019) Leg dominance effect on isokinetic muscle strength of the hip joint. J Chiropr Med, 18: 27-32.
AGUR AMR, DALLEY AF (2016) Grant’s Atlas of Anatomy. Wolters Kluwer.
BASKIN RJ, LIEBER RL (1983) Light diffraction: studies on striated muscle. Trends Biomech, 8: 197-200.
BOTTNER F, PELLICCI PM (2006) Review: posterior soft tissue repair in primary total hip arthroplasty. HSS J, 2: 7-11.
BURKHOLDER TJ, LIEBER RL (2001) Sarcomere length operating range of vertebrate muscles during movement. J Exp Biol, 204: 1529-1536.
CANADIAN INSTITUTE FOR HEALTH INFORMATION (2017) ‘Hip and knee replacements in Canada’. Canadian Joint Replacement Registry Annual Report https://secure.cihi.ca/free_products/cjrr-annual-report-2018-en.pdf
COOK MS, BOU-MALHAM L, ESPARZA MC, ALPERNIN M (2017) Age-related alterations in female obturator internus muscle. Int Urogynecol J, 28: 729-734.
DESILVA JM, ROSENBERG KR (2017) Anatomy, development, and function of the human pelvis. Anat Rec, 300: 628-632.
DOSTAL WR, SODERBERG GL, ANDREWS JG (1987) Actions of hip muscles. Phys Ther, 66: 351-361.
GORDON AM, HUXLEY AF, JULIAN FJ (1966) The variation in isometric tension with sarcomere length in vertebrate muscle fibres. J Physiol, 184: 170-192.
JANSSEN I, HEYMSFIELD SB, WANG Z, ROSS R (2000) Skeletal muscle mass and distribution in 468 men and women aged 18-88 yr. J Appl Physiol, 89: 81-88.
KAWASAKI M, HASEGAWA Y, OKURA T, OCHIAI S, FUJIBAYASHI T (2017) Muscle damage after total hip arthroplasty through the direct anterior approach for developmental dysplasia of the hip. J Arthroplasty, 32: 2466-2473.
LIEBER RL, YEH Y, BASKIN RJ (1984) Sarcomere length determination using laser diffraction: effect of beam and fibre diameter. Biophys J, 45: 1007-1016.
LIEBER RL, FRIDEN J (2001) Clinical significance of skeletal muscle architecture. Clin Orthop Relat Res, 383: 140-151.
MENEGHINI RM, PAGNANO MW, TROUSDALE RT, HOZACK WJ (2006) Muscle damage during MIS total hip arthroplasty: Smith-Petersen versus posterior approach. Clin Orthop Relat Res, 453: 293-298.
MYERS CA, LAZ PJ, SHELBURNE KB, JUDD DL, WINTERS JD, STEVENS-LAPSLEY JE, DAVIDSON BS (2019) Simulated hip abductor strengthening reduces peak joint contact forces in patients with total hip arthroplasty. J Biomech, 93: 18-27.
SACKS RD, ROY RR (1982) Architecture of the hind limb muscles of cats: functional significance. J Morphol, 173: 185-195.
STATISTICS CANADA (2008) ‘Mean height, weight, body mass index (BMI) and prevalence of obesity, by collection method and sex, household population aged 18 to 79, Canada, 2008, 2007 to 2009, and 2005’. http://statcan.gc.ca
SUH KT, PARK BG, CHOI YJ (2004) A posterior approach to primary total hip arthroplasty with soft tissue repair. Clin Orthop Relat Res, 418: 162-167.
WALKER SM, SCHRODT GR (1974) I segment lengths and thin filament periods in skeletal muscle fibers of the Rhesus monkey and the human. Anat Rec, 178: 63-81.
WARD RL, LIEBER RL (2005) Density and hydration of fresh and fixed human skeletal muscle. J Biomech, 38: 2317-2320.
WARD SR, ENG CM, SMALLWOOD LH, LIEBER RL (2009) Are current of lower extremity muscle architecture correct? Clin Orthop Relat Res, 467: 1074-1082.
WINTHER BS, VIGDIS HS, FOSS OA, TINA WS, SVENNINGSEN S, ENGDAL M, HAUGAN K, HUSBY OS (2016) Muscular strength after total hip arthroplasty: A prospective comparison of 3 surgical approaches. Acta Orthop, 87: 22-28.