INTRODUCTION
Iron is an essential metal whose deficiency or excess in the organism is associated with pathologic situations. Iron is clearly hermetic (dose-response relationship), as a small dose of iron is required for human body, the recommended daily intake is 10-15 mg iron/day. Iron is essential in oxygen transport and plays fundamental roles in the oxidative phosphorylation process and other enzymatic functions. Large doses of iron (more than 30 mg/day) produce toxic effects on the liver and brain (Anderson and Shah, 2013; Musacco-Sebio et al., 2014). Systemic iron level depends mainly on iron absorption, storage, and recycling. About 80% of the iron stored in the human body is bound to hemoglobin, with the average of the stored amount in the human body equal to 2–4 gm of iron. In case of iron deficiency, the hemoglobin production decreases leading to anemia, while iron overload causes cell damage (Fung and Nemeth 2013).
The liver has a significant and important role in the metabolic regulation of the iron by production of hepcidin (a key regulating hormone of the entry of iron into the circulation in mammals). Chronic liver diseases affect iron metabolism and result in iron overload. This effect is partially due to low levels of hepcidin. In addition, the accumulated iron causes more damage to the liver by imposing more oxidative stress on hepatocytes (Milic et al., 2016). On the other hand, iron is deposited in acinar cells of the pancreas and in the islets of Langerhans, which in turn may lead to fibrosis and damage of the beta cells resulting in diabetes mellitus (Whittaker et al., 1996). Moreover, Sampaio et al. (2014) stated that iron supplementation in diabetic patients leads to a more oxidative stress that severely affects the pancreatic and the cardiac tissues, denoting that rapid iron supply could produce morphological changes in a relatively short period of time.
NAC is a safe and affordable medication. It is not present in natural sources, although cysteine is present in many food sources like turkey meat, chicken, eggs, yogurt, and garlic (Larsson et al., 2015). NAC is a mucolytic drug which is well tolerated; it modifies the grasping mucous secretions and amplifies glutathione S-transferase activity. The main role of NAC as antioxidant is the stimulation of glutathione (GSH) synthesis, enhancing the detoxification process and acting directly to scavenge the oxygen free radicals (Shahin et al., 2009).
NAC is formed of a small molecule which includes the precursor of reduced GSH and a thiol group. Its clinical efficacy in the treatment of acute heavy metal poisoning had been approved through its protective effect on liver and kidney. It processes antioxidative properties and acts as a chelating agent to eliminate the heavy metals due to its thiol group (Kaplan et al., 2008).
The main target of our study was to investigate the protective role of NAC as a potent antioxidant against the iron toxicity and the resulting induced ferroptosis in the hepatic and pancreatic tissues of male albino rats.
MATERIALS AND METHODS
Experimental animals
Thirty-two male albino rats, weighing 200-250 gm, were used in the present study. Rats were housed in metal cages at room temperature and good ventilation. Rats were fed with standard food-pellets that contained all necessary nutritive elements. They were provided a free access to tap water and were acclimatized for one week prior to the treatment.
Experimental design
Experimental rats were randomly divided into four groups, eight animals each group. For four weeks, the duration of the experiment, the rat groups received treatment as follows: Group I (control group) received a daily single intra-peritoneal injection of 0.9% NaCl (normal saline) (pH: 7.4) in equal volume to iron injection; in Group II (NAC treated group), the rats received NAC (Fluimucil) in a dose of 300 mg/kg by oral gavage according to (Galicia-Moreno et al., 2009); in Group III (iron-treated group), the rats received intraperitoneal injection of iron in a dose of 100 mg/kg (Zhoa et al., 2005), three times per week; in Group IV (NAC and iron-treated group), the rats received NAC in a dose 300 mg/kg by oral gavage daily and intraperitoneal injection of iron in a dose of 100 mg/kg three times per week.
Drugs
Haemojet (Iron): Haemojet ampoules were purchased from a local pharmacy; the drug is produced by Pharco B International Pharmaceutical Company for the European Egyptian Pharmaceutical Industrial Company, Alexandria, Egypt. A pack of three ampoules, each ampoule containing two ml solution with equivalent elemental iron (100mg) as ferric hydroxide polymaltose complex.
Fluimucil (N-Acetylcysteine): Fluimucil tablets (Zambon Switzerland Ltd, 6814 Cadempino, - Switzerland, imported by: Pharma Con Company) were purchased from a local pharmacy, with active constituent is NAC 600 mg. Tablets were dissolved in deionized distilled water, and given to the rats by oral gavage in a dose of 300 mg/kg daily.
Tissue collection
After four weeks, the experimental rats were anaesthetized by isoflurane inhalation and sacrificed. To separate the serum, we collected blood slowly by cardiac puncture, allowing it to clot for 30 min, at least, at room temperature, followed by centrifugation at 2500 rpm for 15 min at 4 °C. The liver and pancreas organs were dissected out and tissues were sampled and processed for different histopathological procedures.
Histopathological procedures
Specimens from liver and pancreas were taken and emerged in 10% buffered formaldehyde solution for at least 24 h for fixation. The specimens were embedded in paraffin wax and 5-μm sections were prepared for histological staining (Slaoui and Fiette, 2011). Prepared slides were stained with hematoxylin and eosin (H&E) stain for an assessment of morphology of the hepatic and pancreatic tissues and observation of the presence, distribution and invasion of the inflammatory cells. Prussian blue (Pb) staining was done for detection of iron deposition in the hepatic and pancreatic tissues (Muñoz et al., 2009). Stained tissue sections slides were examined under a light microscope at 40X magnification.
Electron Microscopic Study
The ultrastructure of the hepatic and pancreatic cells was examined by the use of transmission electron microscopy. To prepare the hepatic and pancreatic tissues, fixation with 2.5% glutaraldehyde in 0.1M phosphate buffer (pH 7.4) overnight at 4 °C was done, with subsequent post-fixation processing in 2% phosphate-buffered osmium tetroxide. The tissues were dehydrated through a serial of graded ethanol, washed in propylene oxide, and embedded in epon resin using EMbed-812 embedding kit. Ultra-thin sections (60–80 nm thick) were done and mounted on copper grids then stained with uranyl acetate and lead citrate (Peerapanyasut et al., 2014, Peerapanyasut et al., 2019). Examination of the ultrastructure of the prepared specimens was done using JEM-2200 FS transmission electron microscope (JEOL- JEM -100 SX electron microscope, Japan, at the electron microscope unit, Mansoura Faculty of Medicine).
Biochemical analysis
Blood samples were collected and serum was separated for biochemical analysis. Quantitative measurements of serum iron level and serum total iron binding capacity were identified using quantitative diagnostic kits, while measuring of the level of serum ferritin was done using enzyme immune assay ELISA kit (Hosseini et al., 2018).
Statistical analysis
Data collected were represented as means ± SEM. Statistical analysis was done by using GraphPad Prism -6, GraphPad Software, San Diego, California. Test used for multiple comparisons between the different groups was One-way ANOVA test. In all tests P value set as <0.05 or less was considered to be statistically significant.
RESULTS
H&E histopathological findings
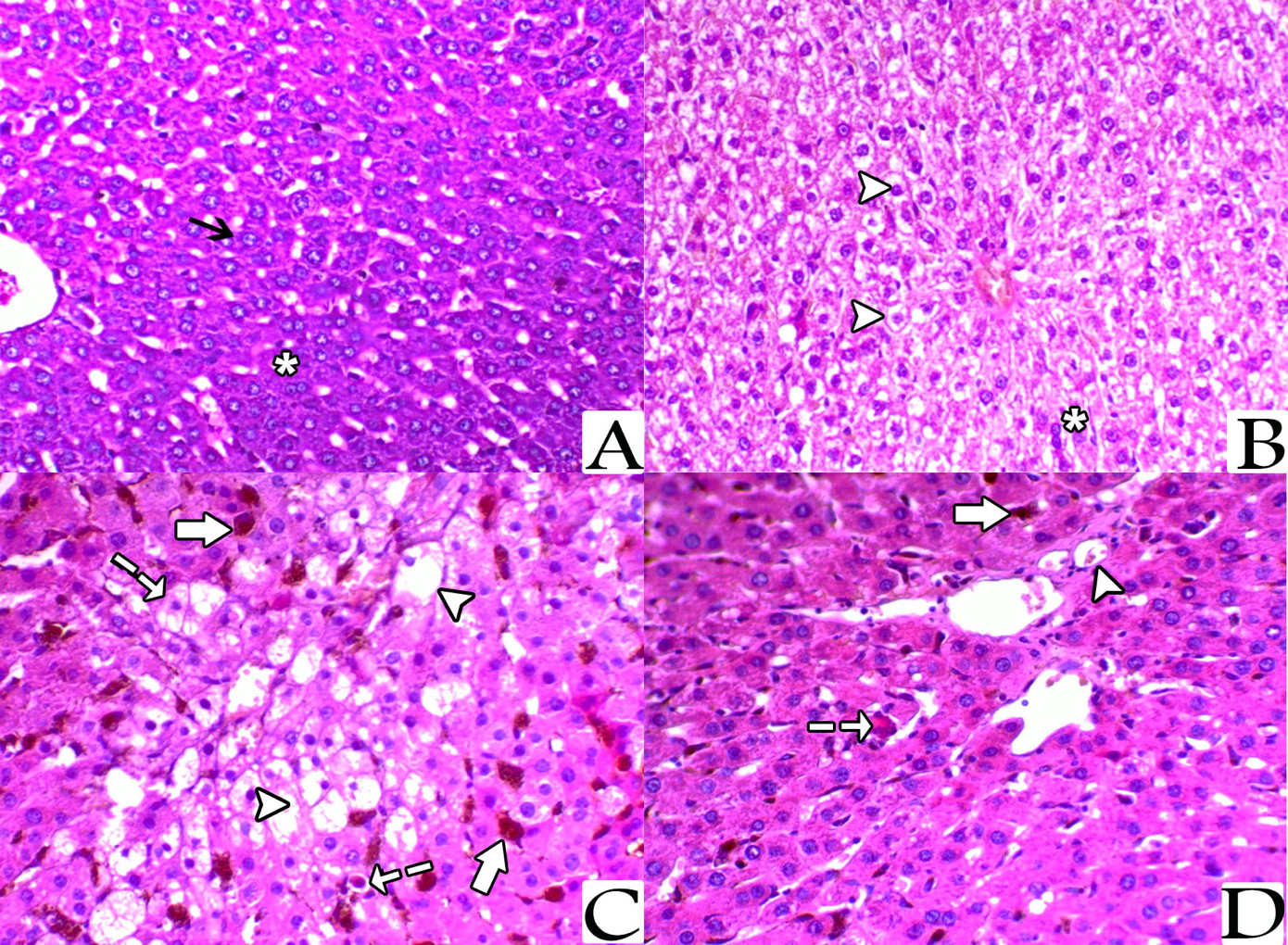
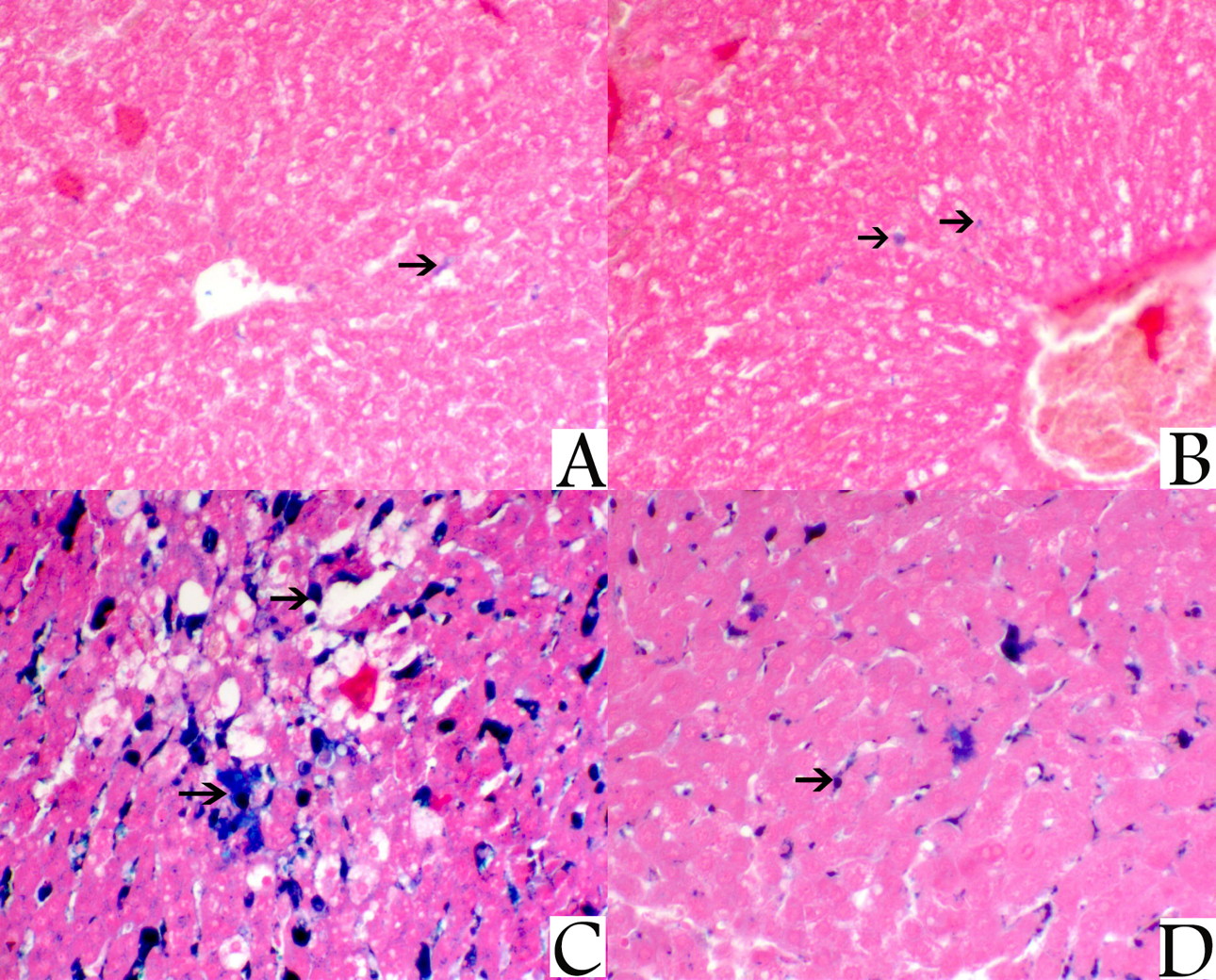
Histopathological examination with light microscope of liver sections from the control rats stained with H&E showed normal architecture of the hepatic lobules with the hepatocytes arranged as cords radiating from the central vein. The hepatocytes cytoplasm appeared strongly granulated, eosinophilic, and with distinct central nuclei. The hepatocytes in the sections from liver of rats treated with NAC showed a mild degree of vacuolization, while sections from the liver of rats of the iron-treated group showed marked hepatic vacuolization mainly with centrolobular distribution, and impacted sinusoids with brownish haemosiderin pigments. Also, there were many hepatocytes revealed, single cell necrosis and apoptosis in this group. Meanwhile, sections from the rats of the last group (NAC + Iron-treated group) showed a marked decrease the hepatic vacuolization, decreased apoptosis, and an obvious clear decrease of the haemosiderin pigments impaction within the blood sinusoids (Fig. 1).
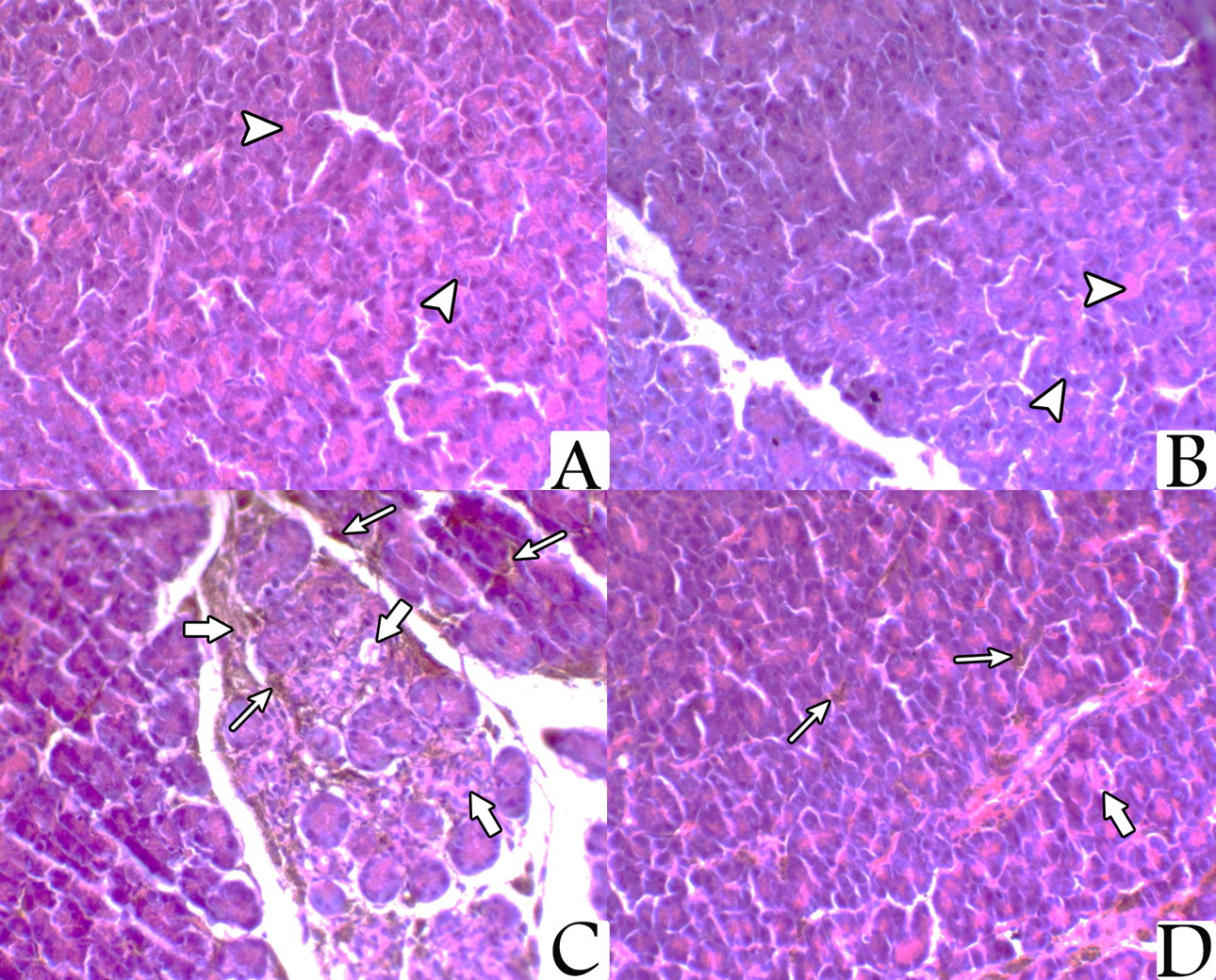
Pancreatic sections from the rats of both control group and NAC treated group showed normal pancreatic architecture composed of serous acini with apical acidophilia and basal basophilia. The nuclei were basal in position, rounded in shape and surrounded by basal basophilic cytoplasm. While sections from the pancreas of the iron-treated rats revealed marked pancreatic acinar degeneration, necrosis, and associated with marked interstitial hemosiderin deposition. However, sections from the pancreas of the rats treated with both NAC and iron at same time showed a mild degree of acinar degeneration and decreased hemosiderin pigments deposition (Fig. 2).
Electron microscopic study
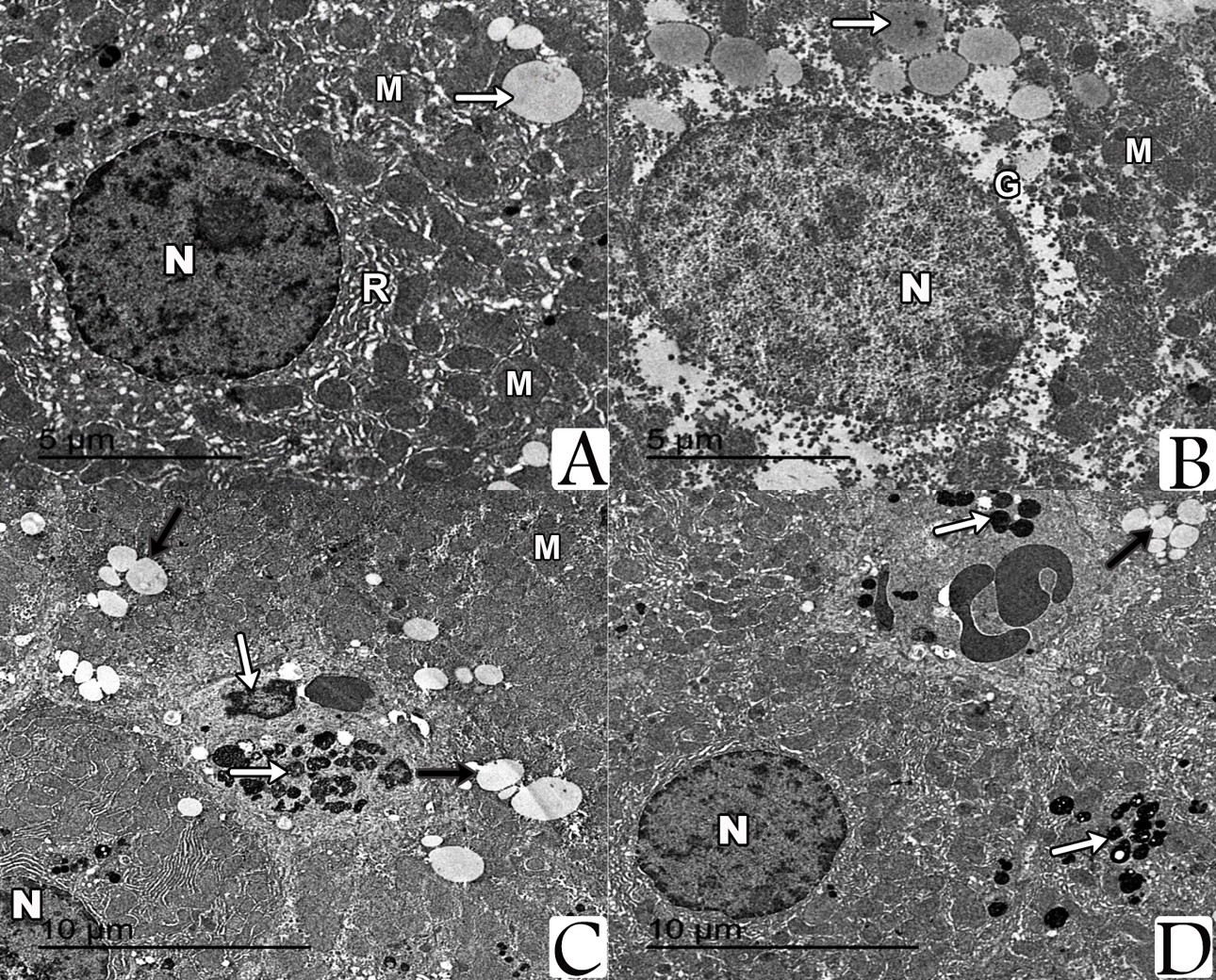
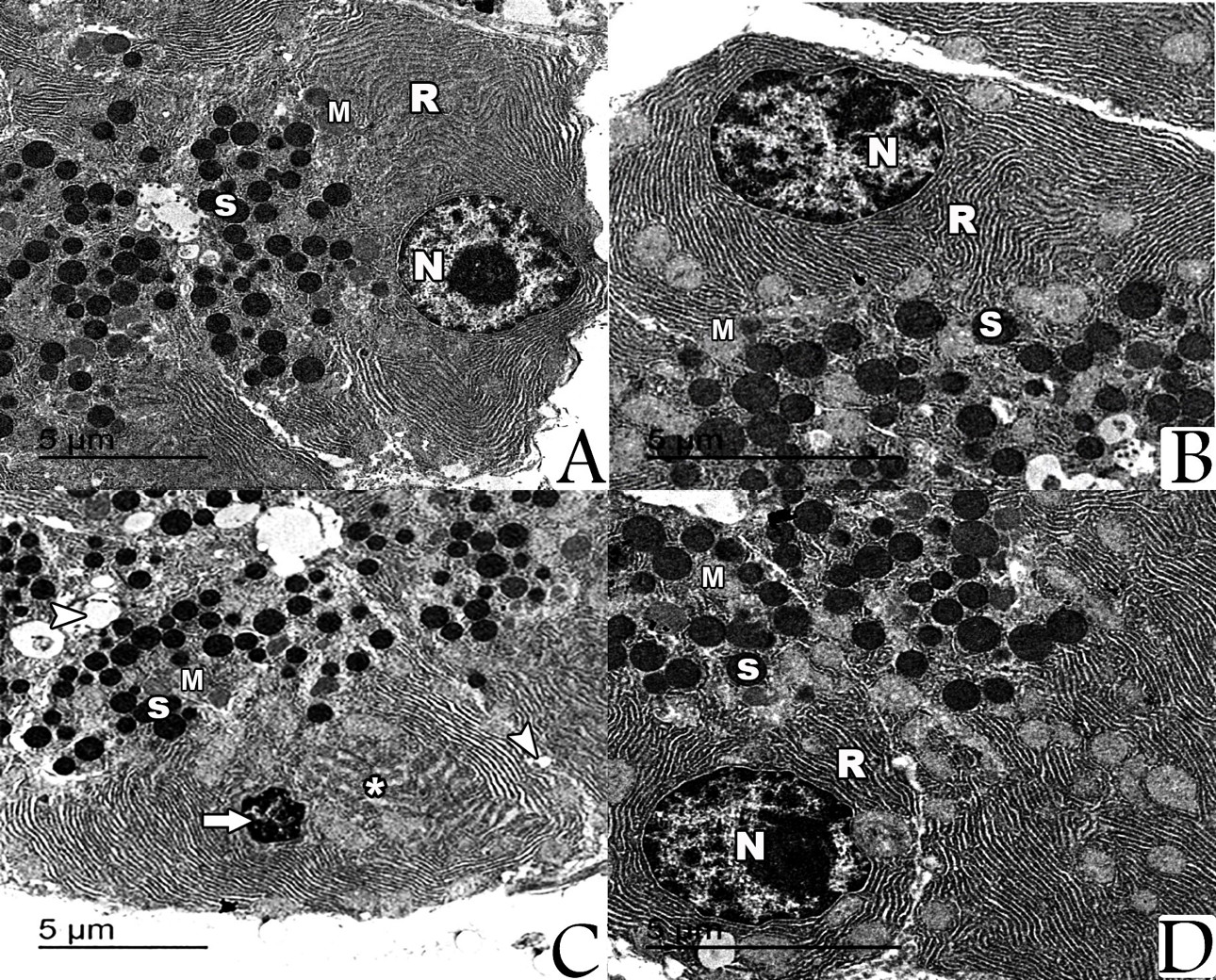
Examination by TEM of sections prepared from the liver of both control group and NAC-treated group showed hepatocytes with their nuclei exhibited regular outlines, prominent peripheral heterochromatin and prominent nucleolus. The cytoplasm showed regular rough endoplasmic reticulum (rER) surrounding the nucleus and many scattered mitochondria with apparent cristae. Multiple glycogen rosettes and few well circumscribed lipid globules were also seen. Sections from the liver of iron-treated rats showed hepatic steatosis associated with deposition of iron particles. On the other hand, ultrastructural examination of hepatocytes from rats treated with both of NAC and iron showed marked decrease of hepatic steatosis associated with decreased iron particles deposition (Fig. 3).
By electron microscope, examination of the prepared pancreatic sections of both control group and NAC-treated rats revealed the well-known normal picture of the exocrine cells of the pancreas. The exocrine acinar cells contained spherical electron dense secretory granules of variable sizes and shape, which were aggregated in the apical cytoplasm called zymogen granules. The acinar cells had large basal nuclei with prominent central nucleoli and dispersed chromatin. Their cytoplasm was characterized by extensive perinuclear rough endoplasmic reticulum arranged in parallel strands and abundant rounded mitochondria, which had regular cristea. Most of the acinar cells from the iron-treated rats’ specimens revealed marked degenerative changes manifested by multiple vacuoles, dilated rER, and the nuclei were small, irregular with condensed chromatin (pyknotic). For the rats that were treated with both NAC and iron, most of the ultrastructural examined sections showed that the pancreatic acinar cells were completely normal and arranged around central lumen making the normal architecture of the pancreatic acini. Each cell had basally located nuclei and apical zymogen granules. The cytoplasm had normal mitochondria and normal rough endoplasmic reticulum. The nuclei were rounded, with central nucleoli, basally located and heterochromatic (Fig. 4).
Prussian blue stain
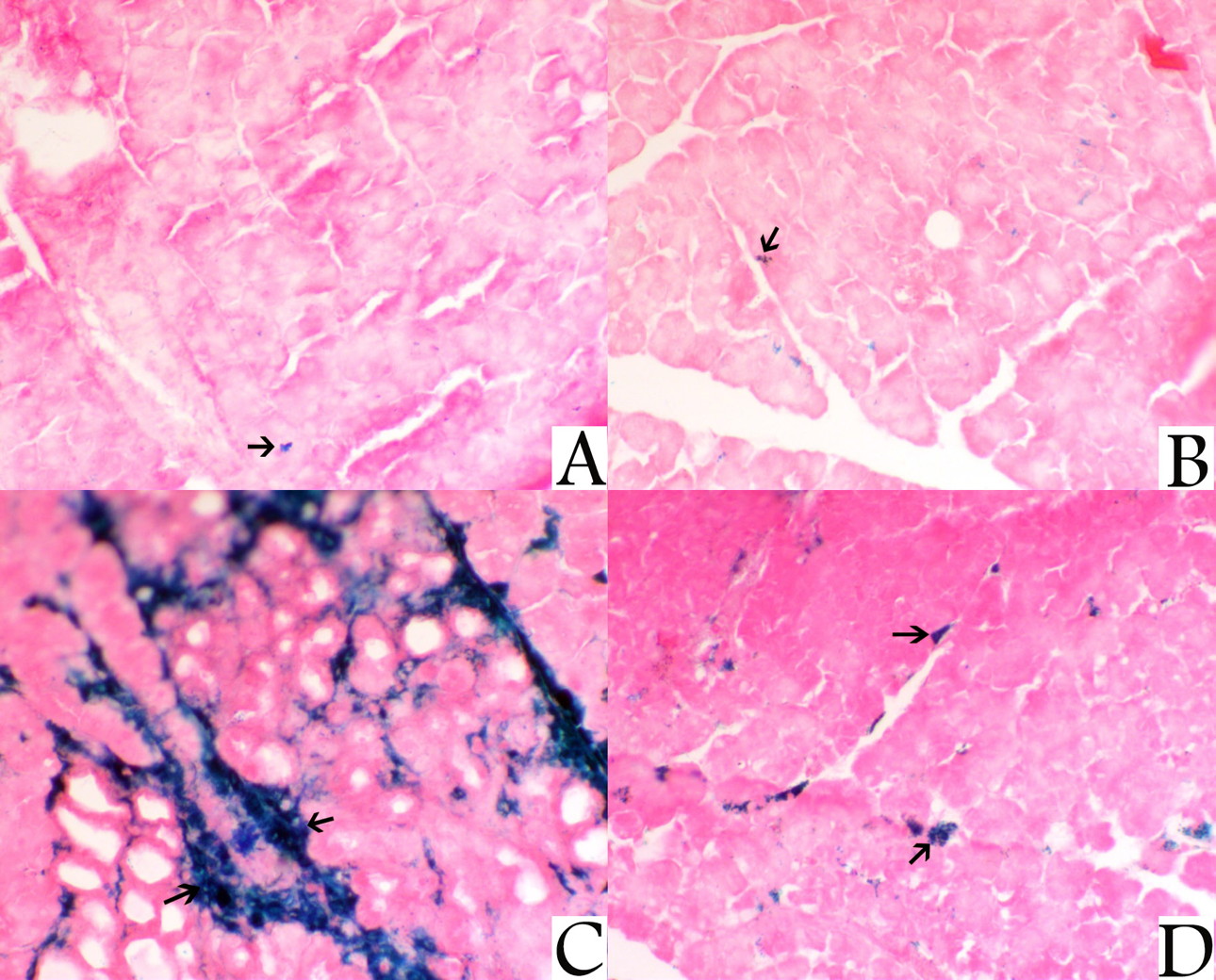
Prussian blue stain was used for the identification of iron in tissues. It is an extremely sensitive stain, and can even detect single granule of iron in cells. On examination of sections from the liver of control rat group and NAC-treated rat group stained with Pb, it showed a mild reactive Pb staining mostly within blood sinusoids. Sections from the liver of iron-treated rats revealed an extensive centrolobular staining with Pb stain, whereas sections of the liver of rats that were treated with bath NAC and iron showed marked decrease of sinusoidal staining with Pb denoting decreased hemosiderin deposition (Fig. 5).
Sections from the pancreas of the control rat group and NAC-treated rat group stained with PB revealed a mild interstitial haemosiderin deposition, while sections from the pancreas of iron-treated rats stained with PB showed marked interstitial haemosiderin deposition. Sections from the pancreas of rat group treated with both NAC plus iron showed a marked decrease Pb-stained area denoting decreased interstitial haemosiderin deposition (Fig. 6).
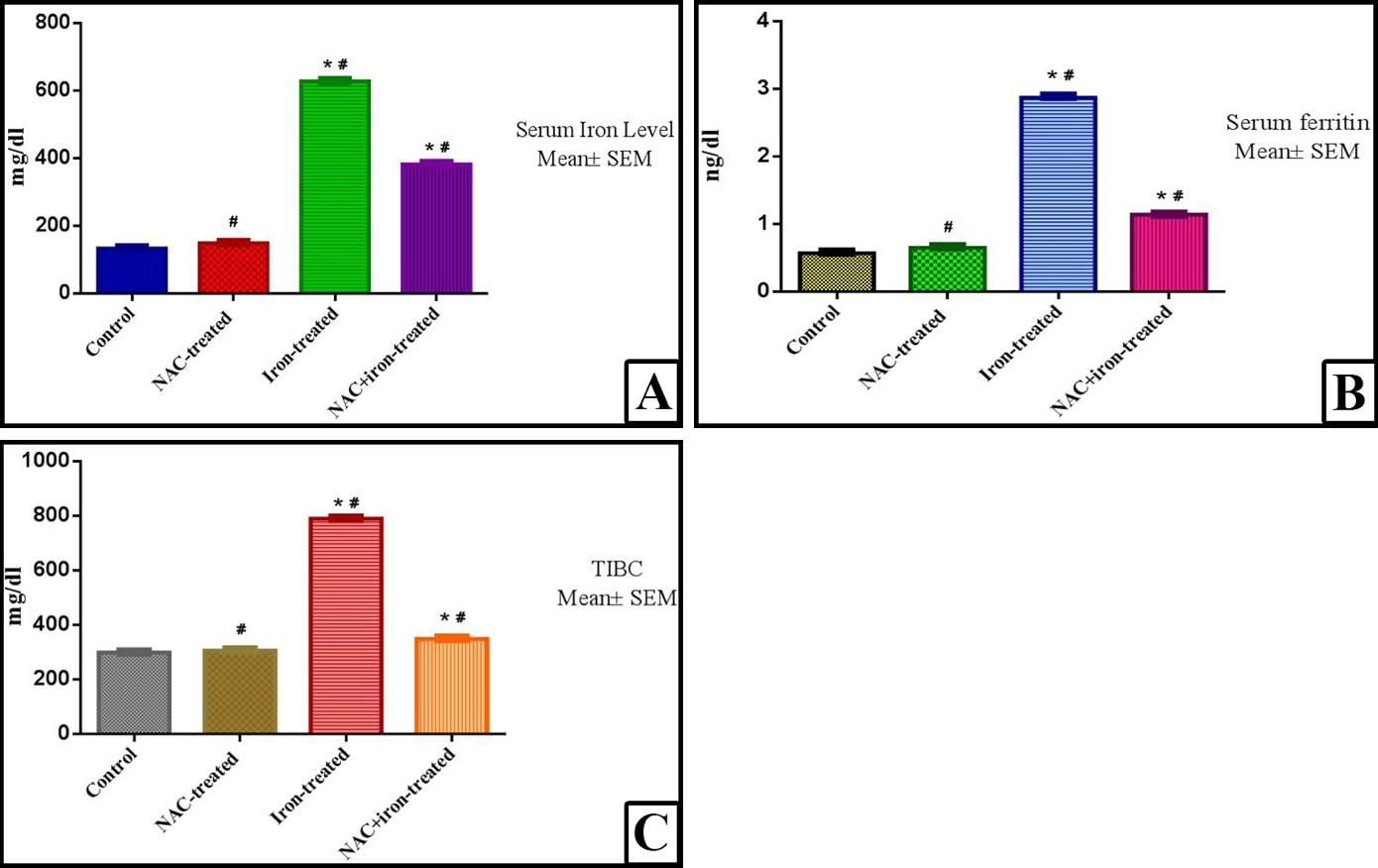
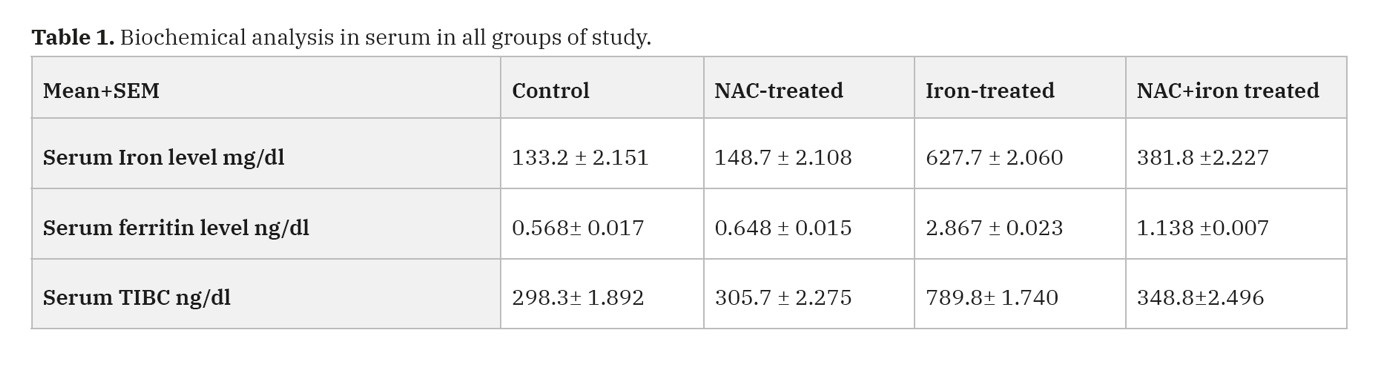
Biochemical analysis (Table 1, Fig. 7)
As regards to the levels of serum iron, in NAC-treated group, there was no significant (p value < 0.001) increase in the levels of serum iron as compared with control group, in iron-treated group there was a highly significant increase in the levels of serum iron as compared with control group (p value < 0.001). In the rats that were treated with combined NAC and iron, there was significant (P value < 0.05) decrease in serum iron level as compared with iron-treated group, but it is still highly significantly increased as compared with the control group (p value < 0.001).
In serum ferritin levels, in NAC-treated group, there was no significant (p value < 0.001) increase in the level of serum ferritin as compared with control group, while the iron-treated group had a highly significant (P value < 0.001) increase in the level of serum ferritin as compared with control group. In the combined NAC and iron-treated group, there was significant (P value < 0.05) decrease in serum ferritin level as compared with the iron-treated group, but still significantly higher than control group too (p value < 0.001).
Regarding the serum total iron binding capacity (TIBC) levels, in the NAC-treated group there was no significant increase in the level of (TIBC) as compared with control group (p value < 0.001). In the iron-treated group there was a highly significant increase in the level of (TIBC) as compared with control group (p value < 0.001). Addition of NAC to iron treatment of the rats, there was a significant (p value < 0.05) decrease in serum (TIBC) level as compared with iron-treated group but it is still highly significant increased as compared with the control group (p value < 0.001).
DISCUSSION
Iron is considered an essential element for many cellular processes in living bodies. Elevated iron in tissue results in several pathological conditions, especially in hepatic function (Fraga and Oteizab, 2002).
Iron overload is usually associated with oxidative stress. Oxidative stress results from excess release of oxygen free radicals, exceeding the antioxidant mechanism capacity. Therefore, current studies should be directed towards establishing novel means to make limitation and decrease the potential iron-dependent damage through decrease and elimination of the formation and release of these free radicals (Puntarulo, 2005).
Excess and acute iron administration is associated with reduction and consumption of the reduced GSH system in hepatocytes and changes in the GSH system enzymes (Abu-Kishk., 2010). GSH plays a key role in regulating numerous cellular activities and helps to keep the immune system. As an antioxidant, it helps to neutralize free radicals that damage cells and tissues at the molecular level. GSH is a powerful antioxidant, is created by binding of amino acid cysteine with two other amino acids glycine and glutamine. NAC form the medical supplement of the amino acid cysteine (Cathy and Richard, 2020).
Ferroptosis is a form of nonapoptotic cell death for which key regulators remain unknown. Depletion of GSH causes inactivation of glutathione peroxidases (GPXs), which is an essential regulator of ferroptotic cell death (Yang et al., 2013). In their study on Sertoli cells and testicular ischeama and reperfusion, Li et al. (2018), reported that ferroptosis is a pervasive and dynamic type of cell death; they interestingly find that depletion of GSH is accompanied with accumulation of iron, and lipid reactive oxygen species (ROS) in addition to blockage of GSH dependent (GPX4) due to GSH depletion. Activation of the GPX4 with availability of GSH blocked the induced ferroptosis by reducing lipid ROS levels and the resulting cell death due to ischeamia that was inhibited by the ROS inhibitor NAC.
In this study, intake of excess iron led to accumulation of granular iron deposits in pancreatic and liver tissues. It was observed that there was a significant difference between iron-treated group and combined NAC and iron-treated group regarding serum iron level, TIBC, and serum ferritin levels, denoting that the addition of NAC had no additional effect on increasing iron absorption from gastrointestinal tract. This is in contrast to Abu-Kishk et al. (2010), who stated that the oral administration of NAC after acute iron toxicity increases the iron absorption from the gastrointestinal tract, leading to higher serum iron levels and hence more liver damage and mortality. Breitbart et al. (2011) and Boveris et al. (2012) discussed that hepatotoxicity induced by acute iron toxicity is caused mostly due to free radical generation and the subsequent lipid peroxidation, as iron helps to induce the creation and liberation of hydroxyl radical, one of the most potent free radicals, which in turn triggers the lipid peroxidation.
Due to high reactivity of the free oxygen radicals, they produce damage to the cells they originate, and their elimination leads to reduced GSH depletion. Due to this affection of GSH system by acute iron toxicity, NAC is suggested as adjuvant treatment. NAC is a well-established potent antioxidant and GSH substitute, which is widely used as an antidote for various intoxications reducing the lipid peroxidation and enhancing the endogenous antioxidant system (Hundekari et al., 2013).
The major findings of this study are as follows: 1) administration of iron for four weeks led to developing of iron overload condition in the rats, shown by an increase in the following: serum iron, TIBC, and serum ferritin, concurrent treatment of the rats with NAC and Iron led to significant decrease in serum iron, TIBC, and serum ferritin compared to iron overloaded groups. 2) NAC treatment with iron overloading exposure significantly improved hepatocytes mitochondrial condition, with decline of the vacuolization process, decreased of both degeneration of acinar cells and vascular and interstitial haemosiderosis. The same results were obtained by Wongjaikam et al. (2016), who reported that NAC provided enhancing effects in reduction of systemic and cardiac iron overload plus decreasing the plasma and cardiac oxidative stress. In turn, administration of NAC resulted in improvement of the cardiac mitochondrial function, cardiac sympathovagal balance and left-ventricular function.
The liver coordinates the balance of the body iron levels by releasing hepcidin, “the iron hormone”. During different physiological conditions, the production of hepcidin is decreased or induced by iron deficiency or by iron loading respectively. Hepcidin stimulates the breakdown of the iron exporter “ferroportin” to decrease the entry of iron into the bloodstream from dietary sources, iron recycling macrophages, and from the different body stores (Wang and Babitt, 2019). There are no known mechanisms to control the excretion of excess iron, hence regulation of the body iron homeostasis takes place at the sites of absorption, utilisation and recycling (Wallace, 2016). Increased TIBC and accumulation of the free iron leads to an increase in the entry of free iron into hepatic cells and beta cells of the pancreas: this is in agreement with study of Wongjaikam et al. (2017), who reported that excess of iron accumulated in cardiac tissue with entry of the free iron into cardiac cells causes increased oxidative stress via Haber-Weiss and Fenton’s reactions. Induction of acute iron overload in rats by a single intraperitoneal injection of iron-dextran (300 mg/kg) or chronic iron overload by injection of 30 mg/kg/daily for 9 weeks led to oxidative damage of heart and liver which was partly attenuated by subcutaneous injection of NAC (150 mg/kg once), in which the ratio of iron dextran to NAC content was 2:1 (Lou et al., 2009).
Our study showed that increased serum iron and ferritin levels resulted in increased hepatic vacuolization, which affected the function and number of the mitochondria, pancreatic nuclear pyknosis and interstitial haemosiderin deposition. This is in agreement with Gao et al. (2010), who reported in his work a mitochondrial swelling in the iron-overloaded rats with mitochondrial depolarization and damage by increased oxidative stress. Also, Avila et al. (2016) discussed the mitochondrial injury and dysfunction within the cardiac cells resulted from increased oxidative stress in iron-overloaded rats.
In the present study, hepatic steatosis was induced by or associated with increased iron and ferritin levels. This was significantly decreased with adjuvant treatment with NAC. As a general role, exogenous iron overloading resulted in hepatic fibrosis. A proof had been reported in a bunch of studies that hepatic iron overload fails to initiate the expected a fibrogenic response. The results of Park et al. (1987), after he had used iron overloading protocol for 12-months through “3% dietary carbonyl iron” administration led to increase in the hepatic iron concentration more than 50 folds only initiate a mild-to-moderate hepatic fibrosis in rats, mild hepatocyte necrosis and leukocyte infiltration. These results were difficult to reconcile with another experiment that was by the same group of investigators using a 14-month iron loading protocol with no significant fibrosis after (Brown et al., 1997).
Another exception for the golden rule of iron overload and fibrogenic response was reported by induction of hepatic overload in C57Bl/6 mice group fed on a diet containing ferrocene throughout 4 months, which led to a 15 folds increase in hepatic iron levels. This regimen resulted in iron deposition in hepatocytes and sinusoidal lining cells, together with appearance of large siderotic nodules in centrilobular region of the liver. The only fibrogenic response histologically proved was predominantly localized to the siderotic nodules. This pattern of fibrosis had been seen also with long-term carbonyl iron feeding (Valerio and Petersen, 2000). Other experiments had identified the newly appeared collagen fibrils in the liver after iron overload via electron microscopy. However, the use of electron microscopy for identification of the collagen fibrils revealed the minimal extent of fibrosis in these studies (Roberts et al., 1993). Das et al. (2016), after the induction of the hepatic iron overload in C57BL/6 mice, reported increases in histological fibrosis accompanied by significant inflammation, an evident that the induction of inflammation may be important in potentiating hepatic fibrosis in iron overloading model.
Conclusion
In our study, iron-overload led to a centrilobular interstitial haemosiderin deposition, hepatic steatosis, sinusoidal impaction of iron particles, mitochondrial dysfunction, nuclear pyknosis, and hepatic and pancreatic increased vacuolization. All these histopathological changes markedly decreased with concomitant treatment with N-Acetyl cysteine to the iron overloaded rats. With the limitation in our study that antioxidant markers should be assessed although we search for the histopathological changes. Further investigations for the detailed mechanism are required.